Targeting cancer metabolism has received interest as a strategy for anticancer therapy. Most cancers have altered metabolism and high demand for nutrients to support their rapid proliferation. There are many other metabolic alterations in cancer cells. They include a high rate of glutamine consumption, enhanced lipid synthesis, and increased pentose phosphate pathway (PPP) flux. Approaches targeting cancer metabolism show promising results in preclinical and clinical studies. In addition, modulation of mitochondrial metabolism that occurs through the tricarboxylic acid (TCA) cycle and electron transport chain (ETC) has a novel approach for anticancer drug development. In this study, Lonidamine inhibits mitochondrial complex II, resulting in alterations in the TCA cycle and glutamine metabolism in the DB-1 melanoma cell line. Lonidamine targets energy metabolism. It mainly involves the inhibition of monocarboxylate transporter (MCT), mitochondrial pyruvate carrier (MPC), resrespiratory chain complex I/II, mitochondrial permeability transition (PT) pore, and hexokinase II (HK-II).
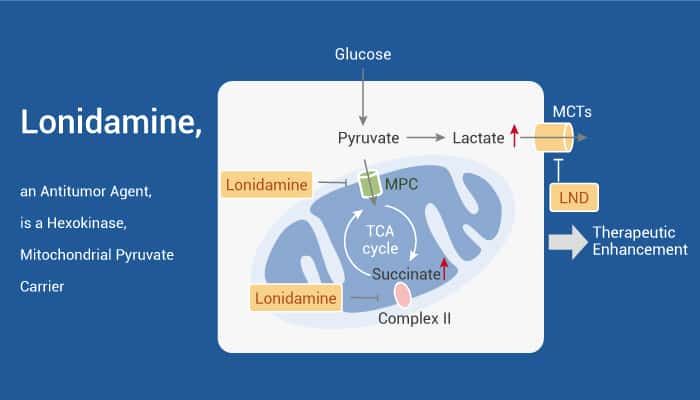
Lonidamine, an antitumor agent, interferes with energy-yielding processes in cancer cells.
Lonidamine (LND) is an indazole derivative. It has antitumor activity by interfering with energy metabolism, especially its action on tumor mitochondria. In addition, Lonidamine inhibits lactate export and the uptake of pyruvate into mitochondria by the inhibition of proton-linked MCT and MPC, respectively. Furthermore, Lonidamine inhibits complexes I and II of the mitochondrial electron transport chain. It also disrupts the mitochondrial transmembrane potential by directly affecting the mitochondrial PT pore. It is under the control of the members of the Bcl-2 family. After treatment, AKT phosphorylation also decreases, which promotes the transfer of p53 from cytoplasm to mitochondria, leading to cell apoptosis. Moreover, Lonidamine is also a chemosensitizer to enhance the antitumor effects of chemotherapeutic drugs, which are based on its disruption of energy metabolism relating to chemo- or radioresistance.
In summary, Lonidamine has antitumor activity by interfering with energy metabolism, especially its action on tumor mitochondria.
Reference:
Huang Y, et al. Cancers (Basel). 2020;12(11):3332. Published 2020 Nov 11.