PARP is a family of proteins involved in many cellular processes, such as DNA repair, genomic stability, and programmed cell death. At least 18 members of the PARP family are encoded by different genes and have homology in conserved catalytic domains. Specifically, PARPs play an important role in various cellular processes. These include regulating chromatin structure, transcription, replication, recombination, and DNA repair.
Besides, PARP1 is a DNA repair enzyme highly expressed in mammalian nuclei. Moreover, PARPi blocks the catalytic activity of the enzyme and has structural similarity with the by-product nicotinamide. Furthermore, PARP inhibitors can induce synthetic lethality in cells deficient in the homologous recombination (HR) pathway. PARP inhibitors also act as radiosensitizers by delaying single-strand break (SSB) repair and leading to subsequent double-strand break (DSB). Meanwhile, PARP inhibitors may also increase the sensitivity of tumors to DNA damaging agents. Therefore, the PARP pathway and its inhibition provide many opportunities for therapeutic intervention in cancer and other disease states. Now, we will introduce a PARP inhibitor, Rucaparib.
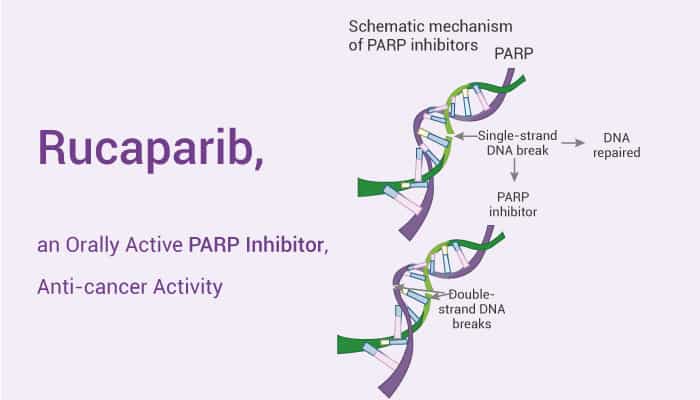
Rucaparib is a PARP Inhibitor.
At first, Rucaparib is an orally active, potent small-molecule inhibitor of PARP proteins (PARP-1, PARP-2, and PARP-3) with a Ki of 1.4 nM for PARP1. Nonetheless, Rucaparib is a modest hexose-6-phosphate dehydrogenase (H6PD) inhibitor. Importantly, Rucaparib has the potential for castration-resistant prostate cancer (CRPC) research.
Secondly, Rucaparib is a possible N-demethylation metabolite of AG14644. Particularly, Rucaparib is cytotoxic and has the LC50 being 5 μM in Capan-1 (BRCA2 mutant) cells and only 100 nM in MX-1 (BRCA1 mutant) cells. The radio-sensitization by Rucaparib is due to downstream inhibition of activation of NF-κB. Obviously, it is independent of SSB repair inhibition. Rucaparib can target NF-κB activated by DNA damage. Rucaparib overcomes toxicity observed with classical NF-κB inhibitors without compromising other vital inflammatory functions. Interestingly, Rucaparib inhibits PARP-1 activity by 97.1% at a concentration of 1 μM in permeabilized D283Med cells
Thirdly, Rucaparib and AG14584 significantly increase Temozolomide toxicity. Additionally, Rucaparib with 0.1 mg/kg results in a 50% increase in the temozolomide-induced tumor growth delay. Rucaparib with 10 mg/kg by i.p. daily for 5 days per week for 6 weeks significantly inhibits the growth of the tumor. In particular, Rucaparib with 150 mg/kg by p.o. once per week for 6 weeks or three times per week for 6 weeks has the greatest antitumor effect. By the way, Rucaparib enhances the antitumor activity of Temozolomide and indicates complete and sustained tumor regression in NB1691 and SHSY5Y xenografts.
Finally, Rucaparib is an orally active, potent PARP inhibitor.
References:
Thomas HD, et al. Mol Cancer Ther, 2007, 6(3), 945-956.
J Murray, et al. Br J Cancer. 2014 Apr 15;110(8):1977-84.