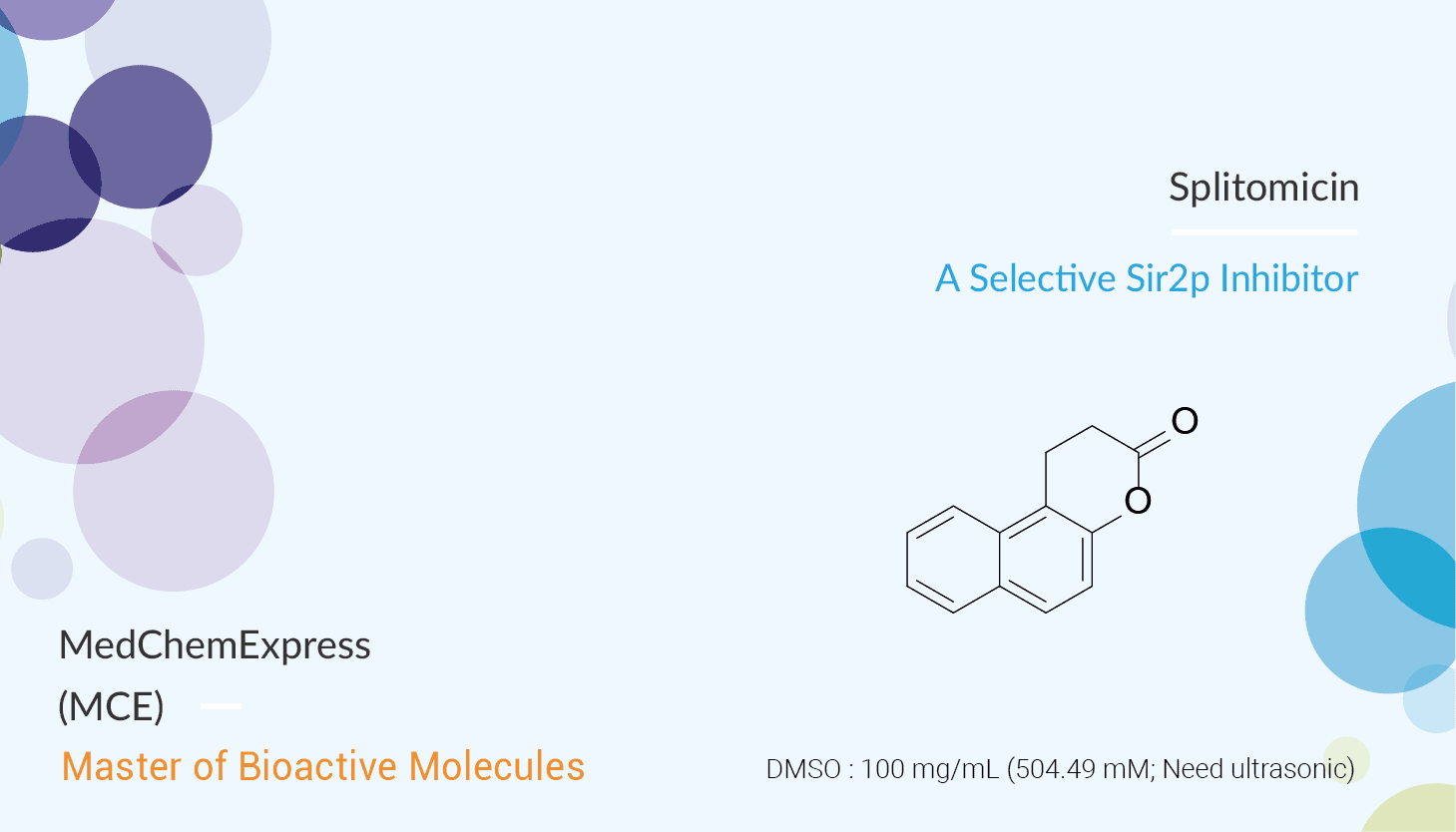
The class III histone deacetylases (HDAC) are also known as the sirtuins. They are a member of the sirtuin family of proteins (SIRT1~7) and homologs of Sir2 (Sir2p) in yeast. SIRT1 shares a domain of about 275 amino acids with other sirtuins. It uses NAD+ to mediate the deacetylation of histone and non-histone proteins and consequently contributes to regulating various cellular functions including control of gene expression, differentiation, metabolism, aging, and tumor suppression. The yeast Sir2 gene is the defining member of a broad family of NAD+-dependent deacetylases in organisms ranging from bacteria to humans. In this study, Splitomicin is a potent inhibitor of Sir2. It is a member of the class III HDAC protein family. Splitomicin inhibits NAD+-dependent HDAC activity of the Sir2 protein. It is a suppressor of SIRT1 and 2, whose biological functions are relevant to genomic stability, DNA repair, p53-mediated apoptosis, and adipogenesis.
 Splitomicin induces dose-dependent inhibition of HDAC in the yeast extract with an IC50 of 60 μM. In vitro, Splitomicin dose-dependently inhibits platelet aggregation and ATP release in washed platelets. Splitomicin also inhibits intracellular calcium mobilization. It inhibits intracellular calcium mobilization. In addition, Splitomicin increases cAMP by inhibiting activity of phosphodiestease. Moreover, Splitomicin (300 µM) attenuated intracellular Ca2+ mobilization, and production of thromboxane B2 (TXB2) in platelets induced by thrombin, collagen, AA, or U46619. In a word, Splitomicin suppresses human platelet aggregation via inhibition of cyclic AMP phosphodiesterase and intracellular Ca2+ release. Furthermore, Splitomicin elicits antiproliferative effects in MCF-7 and H1299 cells in a dose-dependent manner.
Splitomicin induces dose-dependent inhibition of HDAC in the yeast extract with an IC50 of 60 μM. In vitro, Splitomicin dose-dependently inhibits platelet aggregation and ATP release in washed platelets. Splitomicin also inhibits intracellular calcium mobilization. It inhibits intracellular calcium mobilization. In addition, Splitomicin increases cAMP by inhibiting activity of phosphodiestease. Moreover, Splitomicin (300 µM) attenuated intracellular Ca2+ mobilization, and production of thromboxane B2 (TXB2) in platelets induced by thrombin, collagen, AA, or U46619. In a word, Splitomicin suppresses human platelet aggregation via inhibition of cyclic AMP phosphodiesterase and intracellular Ca2+ release. Furthermore, Splitomicin elicits antiproliferative effects in MCF-7 and H1299 cells in a dose-dependent manner.
In summary, Splitomicin, a cell-permeable lactone, is a potent inhibitor of Sir2. Splitomicin inhibits the aggregation of human platelets.
Reference:
Liu FC, et al. Thromb Res. 2009;124(2):199-207.