Microtubules are polymers of tubulin, which is one of several members of a small family of globular proteins. The tubulin superfamily includes five distinct families, the α-, β-, γ-, δ-, and ε-tubulins and a sixth family which is present only in kinetoplastid protozoa. The most common members α-tubulin and β-tubulin, the proteins that make up microtubules. Microtubules are a component of the cytoskeleton, exist in the cytoplasm. Microtubules can grow as long as 50 micrometres, with an average length of 25 µm, and are highly dynamic. The outer diameter of a microtubule is about 23-27 nm, and the inner diameter is about 11-15 nm.
Microtubules are very important in a number of cellular processes. They are play an important role in maintaining the structure of the cell, together with microfilaments and intermediate filaments, they form the cytoskeleton. Microtubules also provide platforms for intracellular transport and are involved in a variety of cellular processes, including the movement of secretory vesicles, organelles, and intracellular macromolecular assemblies. They are also play an important role in cell division and are the major constituents of mitotic spindles.
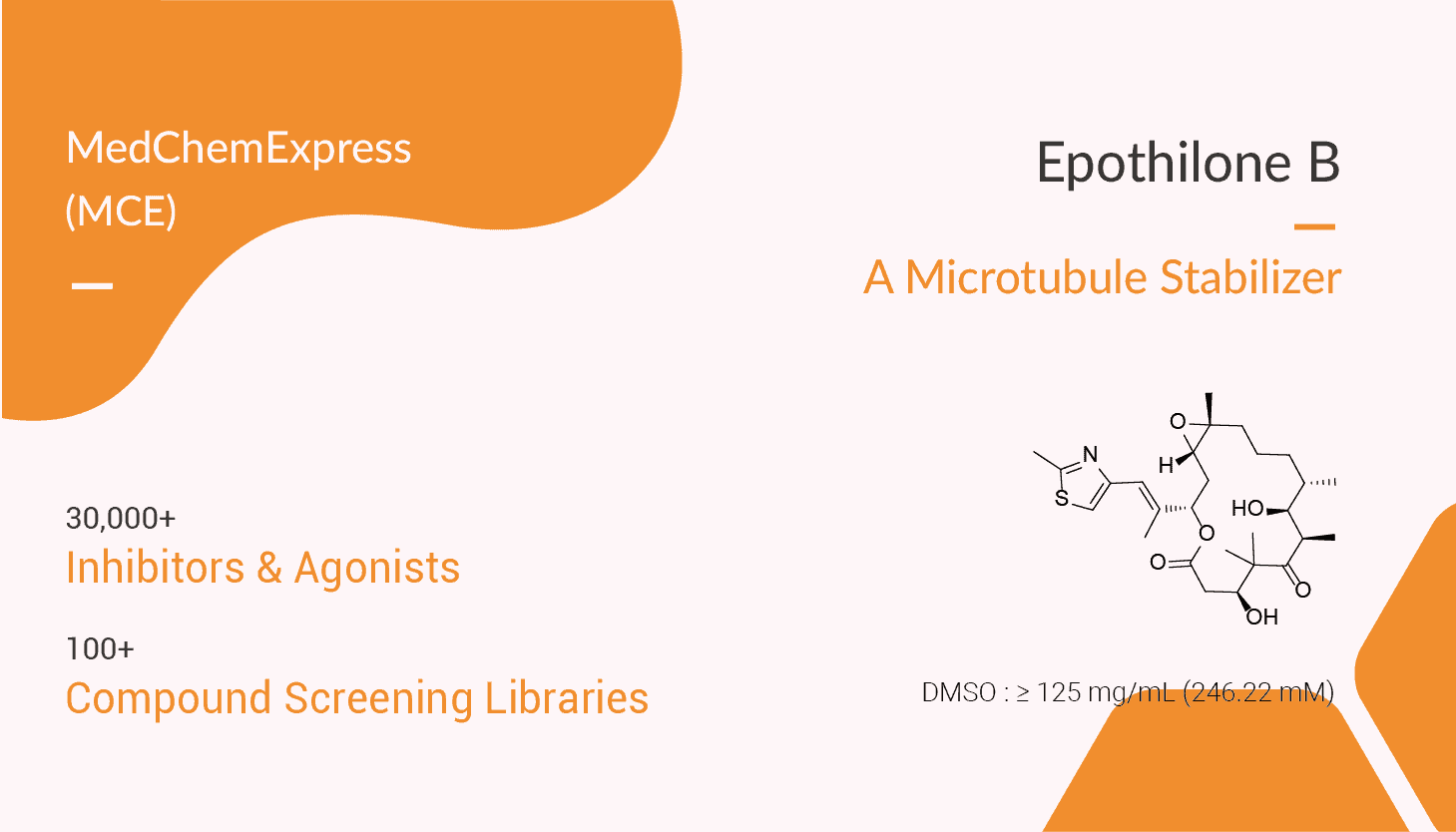
Epothilone B is a non-taxane-related and nonneurotoxic microtubule stabilizer. It acts by binding to the αβ-tubulin heterodimer subunit. Once bound, the rate of αβ-tubulin dissociation decreases, thus stabilizing the microtubules. Furthermore, epothilone B has also been shown to induce tubulin polymerization into microtubules without the presence of GTP. Epothilone B also causes cell cycle arrest at the G2-M transition phase, thus leading to cytotoxicity and eventually cell apoptosis. In vivo, Epothilone B exhibits anti-tumor activity. Treatment with Epothilone B alone results in a partial tumor growth suppression, whereas combined treatment (ionizing radiation) exerts a strong supra-additive tumor growth control.
Reference:
Brouhard JG, et, al. Nat Rev Mol Cell Biol. 2018 Jul;19(7):451-463.