Tiragolumab is a potent TIGIT inhibitor. Tiragolumab alone or in combination with the PD-L1 inhibitor Atezolizumab has effects on solid cancers. Additionally, at two recent medical meetings, the agent achieves statistically significant results in multiple solid malignancies. Especially in small cell lung cancer (NSCLC). Lung cancer is the most commonly occurring cancer in men and third cancer in women.
Lung cancer is the most commonly occurring cancer in men and third cancer in women. What’s more, NSCLC is the most predominant histological subtype. Besides, it represents approximately 85% of all cases of lung cancer.
TIGIT is a receptor, and it expresses in natural killer cells and T cells.
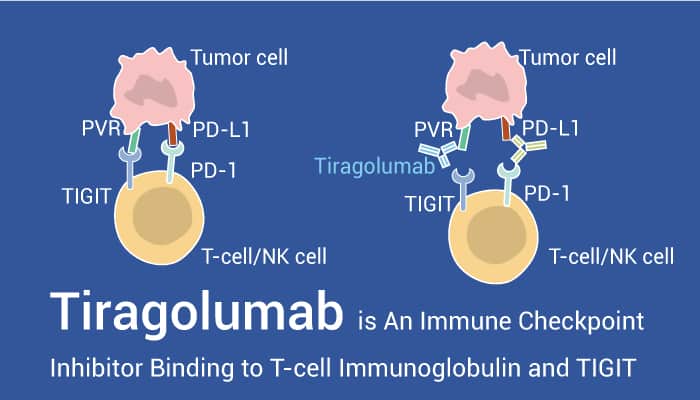
It inhibits immune-cell activity by binding to the PVR ligand on tumor and antigen-presenting cells. Besides, the expression of TIGHT strongly correlates with that of PD-1.
Monoclonal antibodies (mAbs) that block PD-L1 and CTLA-4 have been approved for multiple cancer indications. However, only a subset of patients benefits from immune checkpoint blockade therapies. Thereby, there need other approaches. PD-L1 expression is a hallmark of pre-existing immunity. Besides, it is positively correlated with TIGIT expression.
Tiragolumab is an immune checkpoint inhibitor binding to T-cell immunoglobulin and the ITIM domain (TIGIT). Additionally, Tiragolumab is an immune checkpoint inhibitor binding to T-cell immunoglobulin and ITIM domain (TIGIT).
Tiragolumab is a fully human IgG1/kappa anti-TIGIT monoclonal antibody with an intact Fc region.
In the phase II CITYSCAPE trial, the combination of tira+ Atezolizumab in patients with newly diagnosed PD-L1-positive metastatic NSCLC in the phase II CITYSCAPE trial. As a result, the tumors show high PD-L1 expression (≥50% TPS or tumor proportion score) by the pharmDx immunohistochemistry (IHC) 22C3 assay. Tiragolumab plus Atezolizumab is a promising immunotherapy combination in locally advanced unresectable or metastatic NSCLC.
In conclusion, Tiragolumab is an anti-TIGIT monoclonal antibody for NSCLC research.
Reference:
[1]. Cancer Treat Res Commun. 2020;25:100239.
[2]. Cancer Discov. 2020 Aug;10(8):1086-1087.