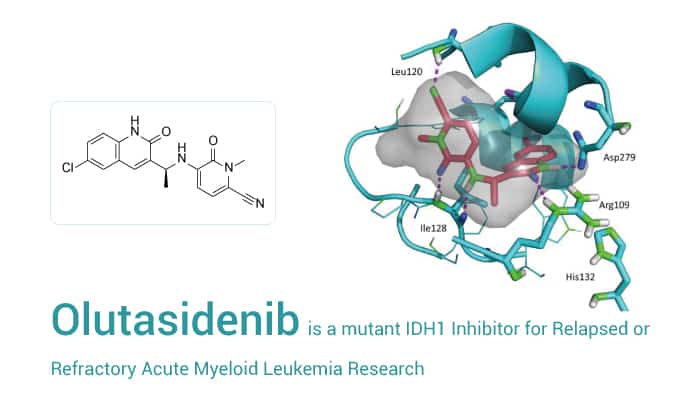
On December 1, 2022, FDA approved Rezlidhea (Olutasidenib) to treat adults with relapsed or refractory acute myeloid leukemia (AML) with a susceptible (IDH1) mutation. Olutasidenib (FT2102) is a highly potent, orally active, brain-penetrant, and selective inhibitor of mutant Isocitrate dehydrogenase 1 (IDH1). As a result, The IC50 values are 21.2 nM and 114 nM for IDH1- R132H and IDH1- R132C, respectively.
IDH1 mutations in cancer lead to abnormal hypermethylation of DNA and suppression of normal cellular differentiation.
In vitro, As a potent IDH1 mutation inhibitor, Olutasidenib is highly selective for IDH1 isoforms, showing no appreciable inhibition against wild-type IDH1 (>20 µM) and IDH2 mutants (> 20 µM). Besides, FT2102 demonstrates a good balance of potency and desirable drug-like properties. In various mIDH1 over-expressing U87 cells and HCT116 cells, Olutasidenib potently inhibits 2-HG production. This means that Olutasidenib could be efficacious against most IDH1-R132 mutant-expressing tumors.
There is a good correlation in potency between cellular assay and in vivo studies.
In the HCT116-IDH1-R132H/+ xenograft bearing female BALB/c Nude mice model, FT2102 exhibits a time and dose-dependent inhibition of 2-HG levels in the tumor.
Furthermore, 50 mg/kg of Olutasidenib inhibits 2-HG levels in the tumor by >90% after the last dose. Calculations based upon the percentage of suppression of 2-HG concentration in tumor versus the free drug concentration in tumor gave in vivo IC50 values of 26 nM and 36 nM in the HCT116-IDH1- R132H or HCT116-IDH1-R132C models, respectively.
Olutasidenib is brain permeable! The brain/plasma ratio of Olutasidenib was found to be 0.38 at the dose of 100 mg/kg. And following a single 100 mg/kg PO dose, the free Cmax concentration of Olutasidenib was ~200 nM, approximately 10-fold of the IC50 value.
All in all, Olutasidenib (Olutasidenib) is an FDA-approved IDH1 mutation Inhibitor. And it has the potential for hematologic malignancies, solid tumors, and CNS tumors research.
Reference:
[1]. J Med Chem. 2020 Feb 27;63(4):1612-1623.