On December 12, 2022, FDA granted accelerated approval to Adagrasib (Krazati), for adult patients with KRAS G12C-mutated locally advanced or metastatic NSCLC. In 2021, FDA approved the selective KRASG12C mutant inhibitor Sotorasib (AMG 510) as a second-line treatment of KRASG12C mutation-positive NSCLC. As a result, Adagrasib (MRTX849) is the second KRASG12C inhibitor to be marketed globally after Lumakras (Sotorasib).
Additionally, FDA also approved the QIAGEN therascreen KRAS RGQ PCR kit (tissue) and the Agilent Resolution ctDx FIRST Assay (plasma) as companion diagnostics for Krazati.
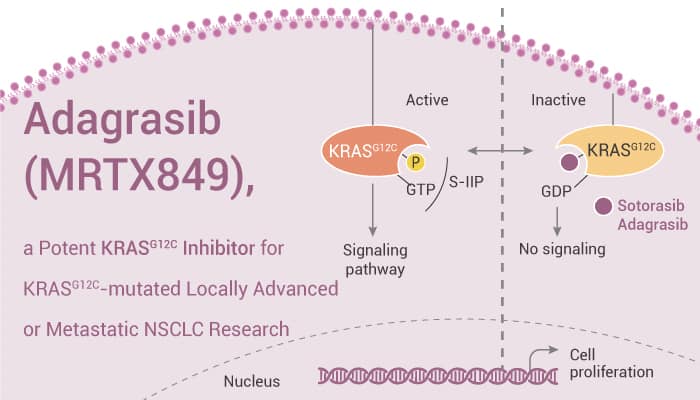
Adagrasib (MRTX849) is a Potent and Selective Inhibitor of KRASG12C.
J Exp Clin Cancer Res. 2022 Jan 19;41(1):27.
It is a potent and orally-available, and mutation-selective covalent inhibitor of KRASG12C with potential antineoplastic activity. Besides, Adagrasib covalently binds to KRASG12C at the cysteine at residue 12, locks the protein in its inactive GDP-bound conformation, and inhibits KRAS-dependent signal transduction.
In vitro, Adagrasib (MRTX849) (0.1-10000 nM; 3-day/2D conditions; 12-day/3D conditions) potently inhibits cell growth in the vast majority of KRASG12C-mutant cell lines. Furthermore, the IC50s range between 10 and 973 nM in the 2D format and between 0.2 and 1042 nM in the 3D format. Adagrasib (MRTX849) also inhibits KRAS-dependent signaling targets including ERK1/2 phosphorylation (Thr202/Tyr204 ERK1; pERK), S6 phosphorylation (RSK-dependent Ser235/236; pS6) and expression of the ERK-regulated DUSP6, each with IC50s in the single-digit nanomolar range in cell lines.
In vivo, In the MIA PaCa-2 model (6-8-week-old, female, athymic nude-Foxn1 nu mice), Adagrasib (i.g.; daily until day 16) demonstrates dose-dependent anti-tumor efficacy over a well-tolerated dose range(1-100 mg/kg). And the maximally efficacious dose of MRTX849 is between 30-100 mg/kg/day. Adagrasib rapidly leads to tumor regression at the earliest posttreatment tumor measurement. Besides, animals in the 30 and 100 mg/kg cohorts exhibit evidence of a complete response on study Day 15. Dosing was stopped on study Day 16, and all 4 mice in the 100 mg/kg cohort and 2 out of 7 mice in the 30 mg/kg cohort remained tumor-free through study Day 70.
In conclusion, Adagrasib (MRTX849) is a Potent KRASG12C Inhibitor for Cancer research.
Reference:
[1]. FDA grants accelerated approval to adagrasib
[2]. Cancer Discov. 2020 Jan;10(1):54-71.