CD47 (Cluster of Differentiation 47) is a transmembrane protein, belonging to the immunoglobulin superfamily. It can interact with signal-regulatory protein alpha (SIRPα) which is an inhibitory transmembrane receptor present on myeloid cells. The interaction leads to different cell-to-cell responses, such as phagocytosis inhibition, cell-cell fusion stimulation, and T-cell activation. Thus, CD47 takes part in a series of cell progresses, including apoptosis, proliferation, invasion, and migration. Besides, it plays a vital role in immune and angiogenesis responses. When it is overexpressed in tumor cells, these cells can avoid being engulfed by phagocytes. It has been proved that the CD47 blockade by antibody efficiently decreased tumor metastasis. Therefore, the block of CD47 is a potent treatment strategy for cancer.
Urabrelimab (SRF231) is a fully human anti-CD47 monoclonal antibody.
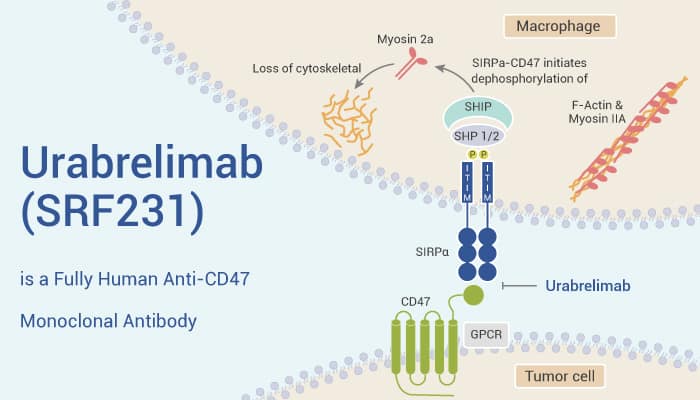
It has a high affinity with CD47 and blocks the CD47-SIRPα interaction, restoring phagocyte engulf CD47+ target tumor cells.
Following the in vitro experiment results, Urabrelimab promotes macrophage-mediated phagocytic clearance of several hematologic primary tumor samples and cell lines. Urabrelimab increases phagocytosis of Raji tumor cell line targets with an EC50 of ~300 ng/mL.
Moreover, in vivo, Urabrelimab administration leads to profound tumor growth inhibition in models of multiple myeloma, diffuse large B cell lymphoma, and Burkitt’s lymphoma as a single agent and in combination with opsonizing antibodies. In the Raji xenograft model, single-agent therapy leads to 112% tumor growth inhibition.
In conclusion, Urabrelimab is a potent anti-CD47 monoclonal antibody, blocking the CD47-SIRPα interaction. It has the potential to research anticancer.
Reference:
1. Holland P M, et al. Blood (2016) 128 (22): 1843.