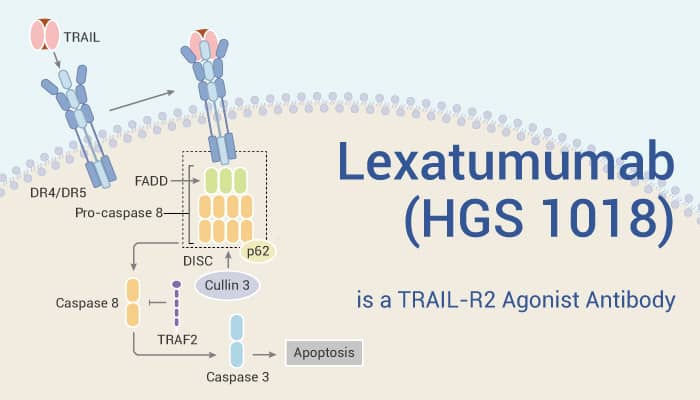
TRAIL-R2 (TNF-related apoptosis-inducing ligand receptor 2), also known as death receptor 5 (DR5), is a cell surface receptor that binds TRAIL, a cytokine that causes apoptosis. Additionally, TRAIL-R2 contains two extracellular cysteine-rich repeats and a cytoplasmic death domain. The receptor engages a caspase-dependent apoptotic pathway. It mediates apoptosis via the intracellular adaptor molecule FADD/MORT1. TRAIL-R2 mRNA is widely present in the spleen, thymus, peripheral blood lymphocytes, and multiple tissues along the gastrointestinal tract. TRAIL-R2 overexpression induces cell death by apoptosis, which not only resulted in membrane blebbing but also induced DNA fragmentation. Therefore, TRAIL-R2 agonism has become a strategy for cancer treatment.
Lexatumumab (HGS 1018) is a TRAIL-R2 agonist antibody.
The combination of Cisplatin with Lexatumumab synergistically inhibits cell growth and enhanced apoptotic death. Lexatumumab (0-10 µg/mL, 72 h) induced various degrees of cell death in the different melanoma cell lines. This antibody (10 mg/kg, IV, twice a week) also increased the antitumor effects in vivo when combined with Dacarbazine. In addition, it induced cleavage of living into its truncated, proapoptotic form, a compound previously shown to accelerate apoptosis.
However, Lexatumumab alone does not show good antitumor effects. In an 8505c thyroid orthotopic model of aggressive thyroid cancer, Lexatumumab (5 mg/kg body weight, IV twice a week) did not result in a statistically significant decrease in tumor volume (76.8±15.2 mm3) or metastasis as compared with control (91.2±20.6 mm3). But when combined with PLX4720 and LY294002, triple-drug therapy significantly increased the levels of apoptotic cells in the tumor and showed dramatic decreases in tumor volume (4.6±3.1 mm3).
In conclusion, Lexatumumab is a potent TRAIL-R2 agonist antibody with antitumor effects but shows stronger efficacy when combined with other anticancer agents.
References:
1. Belyanskaya LL, et al. Mol Cancer. 2007 Oct 22;6:66.
2. Engesæter B, et al. PLoS One. 2012;7(9):e45492.