Glioblastoma is the most frequent and aggressive form of brain tumour. Significantly, the highly invasive growth of these tumors often prevents complete surgical resection. Consequently, patients progress shortly after surgery. In fact, clinical evidence confirms CD95L as a promising novel target for the treatment of recurrent glioblastoma and potentially other malignancies. Moreover, CD95L leads to increased invasion of tumor cells. For this reason, we will introduce Asunercept (APG101), a selective CD95L inhibitor.
Asunercept disrupts CD95/CD95L signaling by selectively binding to CD95L.
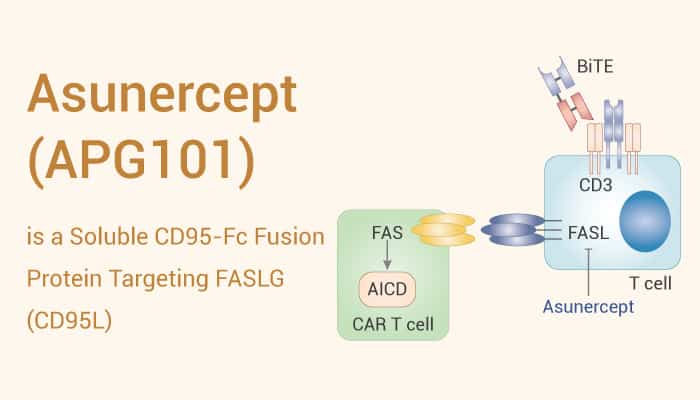
Asunercept is a fusion protein comprising the N-terminal ligand-binding domain of human CD95 and the Fc-part of human IgG1. In addition, Asunercept is designed to interfere with the CD95/CD95L interaction by masking CD95L present on the cell membrane or in soluble form.
In vitro, Asunercept (0-100 µg/mL; preincubation for 30 min) can specifically neutralize the proapoptotic activity of recombinant CD95L in Jurkat A3 cells. Moreover, Asunercept (0.2, 2, 20 µg/mL; 72 h) can interfere with CD95-induced migration/invasion of glioma cells.
In addition, Asunercept offers broad potential applicability to a range of malignancies with aberrant CD95/CD95L signaling, beyond recurrent glioblastoma. Research shows that CD95 is overexpressed on CD34+ progenitors and erythrocytes with myelodysplastic syndrome (MDS). And activation of CD95 is thought to negatively regulate erythrocyte production in the bone marrow. However, direct inhibition of CD95/CD95L signaling plays an important role in rescuing erythropoiesis with potential implications for hematological malignancies such as MDS.
As a selective CD95L Inhibitor, Asunercept is not only effective against glioblastoma, it shows good activity against MDS.
Reference:
- Merz C, et al. Anticancer Drugs. 2015 Aug;26(7):716-27.
- Krendyukov A, et al. Cancer Manag Res. 2019 Sep 2;11:8095-8100.
- Radujkovic A, et al. Cancers (Basel). 2020 Dec 8;12(12):3683.