Breast cancer is subdivided into categories based on the tumor receptor status. Specifically, whether the tumor expresses estrogen receptor (ER), progesterone receptor (PR), or Her2. And, with ER+ disease makes up the majority of patients in the breast cancer patient population. Although there are currently available drugs against ER-positive breast cancers. However, many women eventually relapse with drug-resistant breast cancers. Estrogen and the estrogen receptor (ER) are prominent drivers of breast tumorigenesis and breast cancer progression. Therefore, we will introduce a novel, orally bioavailable, small-molecule selective estrogen receptor degrader-Elacestrant (RAD1901).
Elacestrant is an orally available selective estrogen receptor degrader (SERD) with IC50s of 48 and 870 nM for ERα and ERβ, respectively.
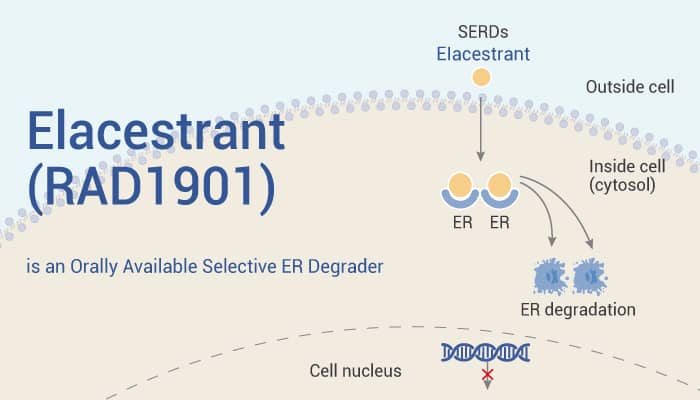
In vitro, Elacestrant (0-1 µM; 24 or 48 h) results in a dose-dependent and marked decrease in estrogen receptor protein expression. In addition, Elacestrant (0.01, 0.1, 1.0 µM) decreases expression of progesterone receptor (PGR, PR; an ER target gene), in both MCF7 and T47D cells. Moreover, Elacestrant (0-1 µM; 48 h) inhibits proliferation of Estradiol (E2)-stimulated MCF-7 cells in a dose-dependent manner, with an EC50 of 4 pM.
In addition, Elacestrant inhibits breast cancer cell proliferation in vivo. Elacestrant (30, 60 mg/kg; p.o.; single daily for 4 weeks) induces complete tumor growth inhibition in MCF7 cell line xenograft model of mice. Interestingly, Elacestrant inhibits the growth of the tumor maintains for 4 weeks after withdrawal. In other words, Elacestrant can produce a stable and continued pharmacodynamic effect on ER signaling and sustained tumor growth inhibition across a range of doses.
Moreover, the combination of Elacestrant with either everolimus or palbociclib resulted in significantly greater tumor growth inhibition as compared with Elacestrant single-agent effects in mice.
To sum up, as a selective ER degrader, Elacestrant is a hopeful agent for breast cancer.
Reference:
- Bihani T, et al. Clin Cancer Res. 2017 Aug 15;23(16):4793-4804.
- Garner F, et al. Anticancer Drugs. 2015 Oct;26(9):948-56.