The B- and T-lymphocyte attenuator (BTLA) is a co-suppressor receptor for B and T cell expression. HVEM (Herpesvirus Entry Mediator), the main ligand of BTLA, also binds to CD160 and LIGHT. HVEM-BTLA is an important checkpoint for Tumor immune escape. Moreover, the couple inhibits the over-activation of lymphocytes and prevents the killing of the immune system. In particular, CD160/BTLA/LIGHT/HVEM constitutes an important signal regulatory network for inflammation, autoimmunity, and infection immunity.
Suppressing BTLA expression leads to the overactivation of T cells and promote disease progression. Here we will introduce a humanized BTLA monoclonal antibody, Tifcemalimab, with a significant effect on lymphoma.
Tifcemalimab is a humanized monoclonal antibody against BTLA, which blocks HVEM-BTLA interaction and activates T-cell signaling.
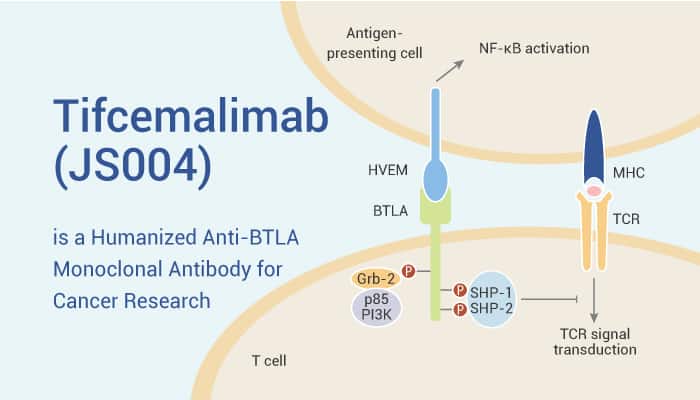
Tifcemalimab is well-tolerated and safe. It targets BTLA and blocks HVEM-BTLA interaction. Therefore, Tifcemalimab blocks the inhibitory signaling pathway mediated by BTLA. BTLA has similarities to PD-1 and CTLA-4, so BTLA also can be used for dual immunotherapy with Toripalimab (an anti-PD-1 antibody).
Relapsed/refractory (R/R) lymphoma is a major challenge in the field of tumor therapy. Moreover, immune checkpoint PD-1/PD-L1 inhibitors exhibit inhibition on relapsed or refractory classic Hodgkin’s lymphoma (R/R cHL). However, there is a lack of other R/R lymphoma inhibitors with equally effective.
Specifically, Tifcemalimab-Toripalimab has strong inhibition in relapsed or refractory lymphomas resistant to PD-1/L1 and/or CD30. They have no dose-limiting toxicity in either single-agent or combination. Immune-related Adverse Events (irAE) are characterized by fever (27.1%) and anemia (25.0%). In terms of clinical antitumor activity, Tifcemalimab monotherapy showed 1/25 partial response (PR) and 7/25 disease stabilization (SD) in follicular lymphoma models. The objective response rate (ORR) and disease control rate (DCR) against the PD-1 antibody model in the combination group reached 39.3% and 85.7% respectively.
In conclusion, Tifcemalimab is a promising monoclonal antibody against BTLA for the study of relapsed/refractory (R/R) lymphoma.
Reference:
[1] Jun M, et, al. Blood (2022) 140 (Supplement 1): 3716–3717.
[2] Yu X, et al. Front Immunol. 2019 Mar 29;10:617.