Melanoma is an immunologic malignancy characterized by higher prevalence in immune-compromised patients. In addition, once melanoma has spread, this type of cancer rapidly becomes life-threatening. Moreover, Melanoma is the ninth most common malignancy and the second for mortality. According to the statistics, cutaneous melanoma causes 55 500 deaths annually. However, for more than 40 years, few treatment options were available, and clinical trials during that time were all unsuccessful. Research showed that melanoma patients with distant metastases show a 5-year survival rate of 23%, making metastasis the leading cause of melanoma-associated deaths. However, how to deal with metastatic melanoma? Now, we will introduce Encorafenib (LGX818), a highly potent BRAF inhibitor for BRAFV600E melanoma.
Encorafenib is a highly potent BRAF inhibitor with selective anti-proliferative and apoptotic activity in cells expressing BRAFV600E (EC50=4 nM).
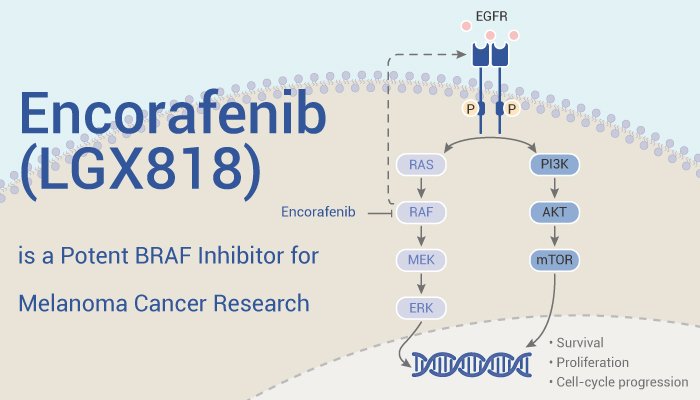
As a potent active small molecule, Encorafenib can prevent diseases or disorders associated with abnormal or deregulated kinase activity, particularly diseases or disorders active modulator that involve abnormal activation of BRaf
In vitro, Encorafenib (10 nM; 12 days) treatment potently inhibits colony formation in A375, G361, and SK-MEL-24 cells, but not in RPMI7951 and C8161 cells. In addition, Encorafenib (10 nM; 72 h) induces senescence in BRAFV600E melanoma cells through p27KIP1 expression and pRb activation.
In addition to inducing senescence, Encorafenib has been shown to promote autophagy in melanoma cells. Research exhibited that Encorafenib (1, 10, 100 nM; 24 h) induces autophagy by inhibiting the mTOR pathway in BRAFV600E melanoma cells. Moreover, Encorafenib treatment induces a steady increase in the β-catenin level in G361 cells over time.
In summary, Encorafenib has potent anti-melanoma effects via the induction of senescence, which is accompanied by autophagy. Encorafenib is a hopeful agent for anti-melanoma.
Reference: