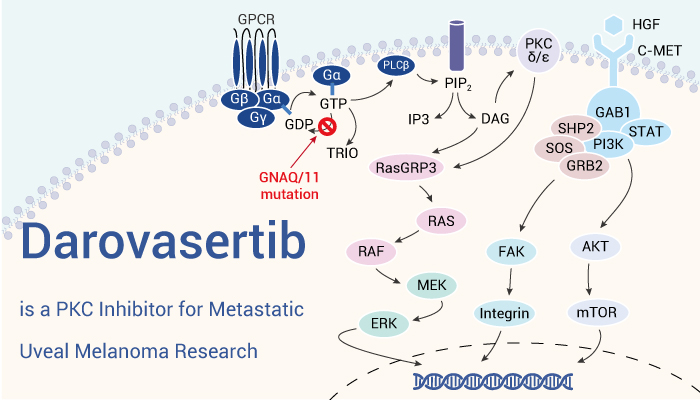
Uveal melanoma (UM) is a common type of primary intraocular cancers. Besides, primary uveal melanoma has high risk of progressing to metastatic uveal melanoma with a poor prognosis. Therefore, it’s important to develop anti-uveal melanoma agent. Furthermore, the activation of the PKC and MAPK pathways play an essential role in the pathogenesis of uveal melanoma. Compared to other PKC inhibitors (such as Sotrastaurin and Enzastaurin), Darovasertib is more potent in inhibiting conventional (α, β) and novel (δ, ϵ, η, θ) PKC proteins, and has a better tolerability and safety profile. Simultaneously, Darovasertib can inhibits PKC and suppresses the downstream signaling cascade, thereby decreasing tumor cell proliferation and survival. In addition, the FDA granted Darovasertib for the treatment of uveal melanoma in May 2022.
Darovasertib, an orally active PKC inhibitor, can be used for uveal melanoma research.
In vitro, Darovasertib inhibits PKC α, β 1, β 2, γ, δ, η, ϵ, θ with IC50s of 25.2, 66, 58, 109, 6.9, 13.3, 2.9, 3.0 nM respectively. In cells, Darovasertib (1 nM-1 μM) displays UM-specific cell viability inhibition activity in OMM1.5, OMM1.3, Me202, 92.1 cells. Besides, Darovasertib (0-1 μM) inhibits phosphorylated FAK, PKN and ERK in 92.1 UM cells. Thus, Darovasertib (0-2 μM, 48 h) together with FAK inhibitor (VS-4718) (0-1 μM) can also promote cytotoxic cell death in vitro.
In vivo, Darovasertib shows low to moderate clearance in mouse, rat, and dog species with good oral bioavailability. Besides, Darovasertib shows rapid absorption in rat and dog with a Tmax of 0.3 and 0.5 h, respectively. Importantly, Darovasertib (15-150 mg/kg, p.o.) inhibits tumor growth in the 92.1 tumor xenograft model in mice. And it reduces phosphorylated tumor levels of PKCδ after a single oral dose.
In conclusion, Darovasertib is a potent and orally active PKC inhibitor, and can be used for uveal melanoma research.
References:
[1] Visser M, et al. J Med Chem. 2024 Jan 25;67(2):1447-1459.
[2] Cao L, et al. Front Pharmacol. 2023 Jul 28;14:1232787.
[3] Arang N, et al. Cell Rep Med. 2023 Nov 21;4(11):101244.