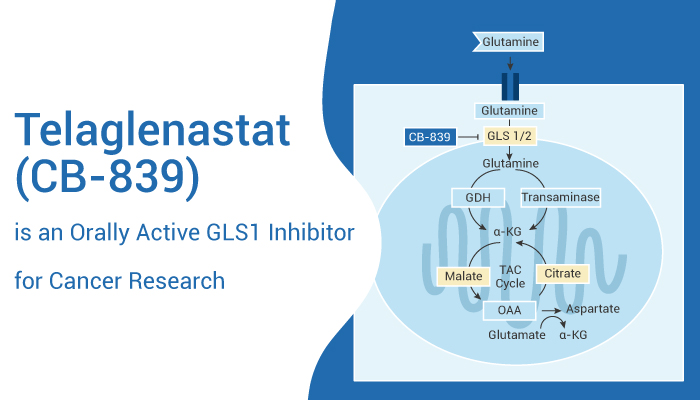
Telaglenastat (CB-839) is a first-in-class, selective, reversible, and orally active Glutaminase-1 (GLS1) inhibitor. Telaglenastat selectively inhibits GLS1 splice variants KGA (kidney-type glutaminase) and GAC (glutaminase C) compared to GLS2. The IC50s are 23 nM and 28 nM for endogenous glutaminase in mouse kidneys and brains, respectively. Telaglenastat induces autophagy and has antitumor activity[1].
In HCC1806, MDA-MB-231 cells, Telaglenastat exhibits antiproliferative activity. Additionally, The IC50 values cells are 49 nM and 26 nM, respectively. Additionally, this inhibitor also induces apoptosis. Telaglenastat (CB-839) (1 μM; 72 hours) activates caspase 3/7 at 1 μM after 72 hours.
In Female nu/nu mice with TNBC patient-derived xenograft model, As a result, Telaglenastat suppressed tumor growth by 61% relative to vehicle control at the end of the study.
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive disease with a poor prognosis. Furthermore, The driving oncogene in PDAC results from the Kras mutation. The oncogenic Kras can promote a metabolic rewiring of PDAC, including the non-canonical use of glutamine (Gln) to support proliferation through redox homeostasis. Telaglenastat is a potent GLS inhibitor. Additionally, GLSi is a potential method for PDAC therapeutic strategy.
Telaglenastat (CB-839) exhibits a potent effect on the proliferation of multiple PDAC cell lines. Besides, the IC50s in the low nanomolar range, show 1.01 nM and 1.73 nM for PaTu-8988T and MPDAC-4 cells, respectively. Additionally, Telaglenastat (CB-839) significantly reduces PDAC growth across multiple cell lines in a multi-day proliferation assay.
Telaglenastat also induces autophagy. it also acts as a potent splice variant of GLS inhibitor.
As a result, it inhibits the effects of Gln and estrogen on UECC’s growth and autophagy in vitro. In Ishikawa and KLE cells, Telaglenastat (CB-839) decreased the expression of Beclin-1, and LC3B, and up-regulated the expression of p62.
In conclusion, Telaglenastat (CB-839) is a potent (GLS1) inhibitor for PDAC research.
Reference:
[1] Gross MI, et al. Mol Cancer Ther. 2014 Apr;13(4):890-901.
[2] Biancur DE, et al. Nat Commun. 2017 Jul 3;8:15965.
[3] Zhou WJ, et al. Cell Commun Signal. 2019 Aug 20;17(1):99.