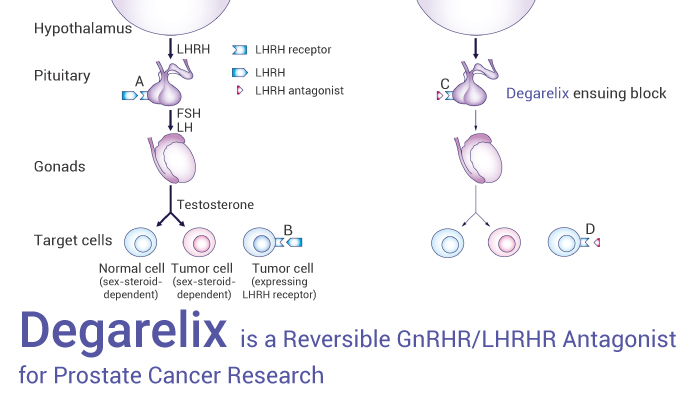
GnRHR (gonadotropin-releasing hormone receptor) is also known as the luteinizing hormone-releasing hormone receptor (LHRHR). Importantly, GnRHR associates with G-proteins that activate a phosphatidylinositol (PtdIns)-calcium second messenger system. Particularly, Activation of the GnRHR ultimately causes the release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH). However, GnRH antagonists produce a rapid and effective suppression of gonadotropin release and therefore gonadal steroid secretion in human males.
In this article, we will introduce a competitive and reversible GnRHR/LHRHR antagonist, Degarelix.
Degarelix (1 nM-10 μM, 0-72 h) reduces cell viability in all prostate cell lines (WPE1-NA22, WPMY-1, BPH-1, VCaP cells), except the PC-3 cells. Besides, Degarelix (10 μM, 0-72 h) exerts a direct effect on prostate cell growth through apoptosis. Specifically, Degarelix induces a significant increase in caspase 3/7 activation.
Degarelix (0-10 μg/kg; s.c.; once) produces a dose-dependent and reversible decrease in plasma LH levels with a minimal effective dose of 3 μg/kg. For the 50 μg/kg and 200 μg/kg doses, t1/2 of absorption values were 4 min and 30 min, Tmax values were 1 h and 5 h, and apparent plasma disappearance t1/2 values were 12 h and 67 h, respectively. Meanwhile, Degarelix Produces a dose-dependent decrease in plasma testosterone levels with a minimal effective dose of 1 μg/kg. Moreover, Degarelix is stable when incubated in microsomes and cryopreserved hepatocytes from animal liver tissue. In rats and dogs, most of the degarelix dose is eliminated within 48 h via urine and feces in equal amounts (40–50% in each matrix). In contrast, in monkeys, the primary route of excretion is fecal (50%) and renal (22%).
All in all, Degarelix is a competitive and reversible gonadotropin-releasing hormone receptor (GnRHR/LHRHR) antagonist and can be used for prostate cancer research.
Reference:
[1] Sakai M, et al. PLoS One. 2015 Mar 26;10(3):e0120670.
[2] Broqua P, et al. J Pharmacol Exp Ther. 2002 Apr;301(1):95-102.
[3] Sonesson A, et al. Drug Metab Dispos. 2011 Oct;39(10):1895-903.