Idiopathic pulmonary fibrosis (IPF) is a specific form of chronic, progressive fibrosing interstitial pneumonia of unknown cause that occurs primarily in older adults. In addition, IPF is characterized by substantial aggregation of myofibroblasts and excessive accumulation of extracellular matrix (ECM), leading to the formation of fibroblastic foci that are interspersed and constantly expanding in the lung interstitium, and has a poor prognosis. However, after diagnosis, the median survival time of IPF patients is only 3-5 years. Hence, we will introduce a pan-Trk/ROS1/ALK inhibitor Entrectinib. Entrectinib not only shows good activity against IPF, but also against cancer. Because ALK, ROS1 and Trk play an important role in cancer.
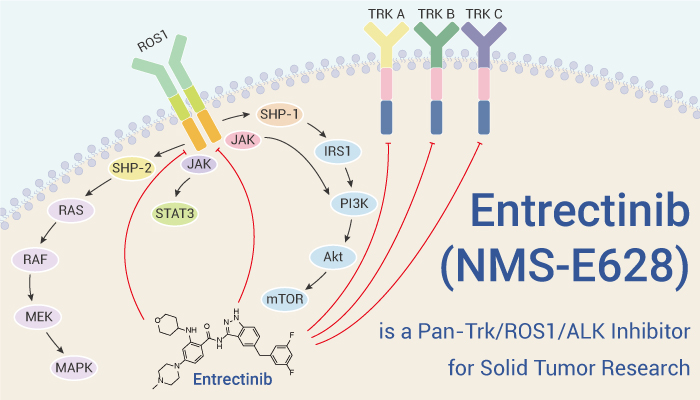
Entrectinib is a pan-Trk/ROS1/ALK inhibitor, with IC50 values of 1, 3, 5, 12 and 7 nM for TrkA/B/C, ROS1 and ALK, respectively.
In vitro experiments, Entrectinib (100, 200, 400 nM; 24 h) alleviates TGF-β1-induced activation of mouse lung fibroblasts. In addition, Entrectinib(100, 200, 400 nM; 24 h) inhibits TGF-β1-induced epithelial to mesenchymal transition of mouse lung epithelial cells. Moreover, Entrectinib (10, 50, 250 nM; 2 h) abolishes autophosphorylation of TPM3-TRKA, concomitant with complete inhibition of the phosphorylation of PLCg1, AKT, and MAPK in KM12 cells. Besides, Entrectinib (10, 50, 250 nM; 24, 48 h) induce KM12 cells cycle arrest (24 h) and apoptosis (48 h).
Furthermore, Entrectinib (20, 40, 60 mg/kg; i.g.; single daily for 7 days) attenuates bleomycin-induced pulmonary fibrosis in mice. Entrectinib also shows good anticancer activities in vivo. For instance, Entrectinib (30, 60 mg/kg; p.o.; twice daily for 10 consecutive days) induces regression of xenograft tumors in tumor-bearing mice, including TRKA-dependent colorectal cancer KM12, ROS1-driven tumors, and several ALK-dependent models of different tissue origins, including brain-localized lung model cancer metastasis.
All in all, Entrectinib is a promising pan-Trk/ROS1/ALK inhibitor for IPF and cancer research.
[1] Miao Y, et al. Int Immunopharmacol. 2022 Dec;113(Pt B):109427.
[2] Ardini E, et al. Mol Cancer Ther. 2016 Apr;15(4):628-39.
[3] Iyer R, et al. Cancer Lett. 2016 Mar 28;372(2):179-86.