KIT receptors belong to the Class III receptor tyrosine kinase (RTK) family, which also includes PDGFRA (platelet-derived growth factor receptor A) and CSF1R (colony stimulating factor 1 receptor). Stem cell factor (SCF) activates KIT by inducing dimerization, autophosphorylation, and initiating downstream signaling. However, KIT is prone to multiple drug-resistant mutations that occur within the kinase’s 5′ -adenosine triphosphate (ATP) binding pocket (exons 13 and 14) or activating ring (exons 17 and 18). In particular, KIT exon 17 mutants confer ligand-independent constitutive kinase activity. The mutants drives disease in solid tumors and hematologic malignancies. Furthermore, KIT mutants are continuously activated, so it is of great significance to explore efficient and selective small molecule inhibitors to inhibit KIT-driven tumors. An orally effective KIT inhibitor is described here.
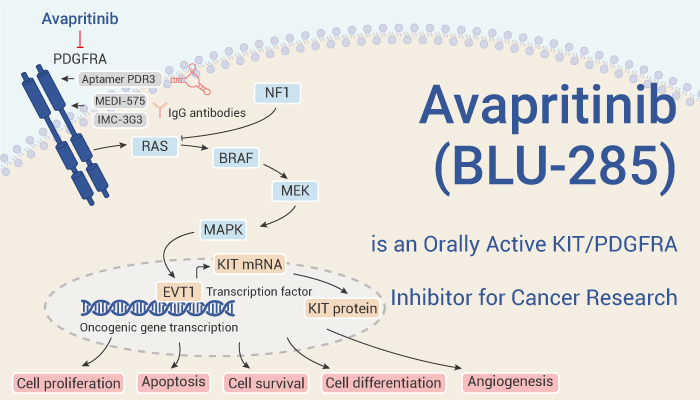
Avapritinib (BLU-285) targets a variety of KIT mutants and has antitumor activity in vivo and in vitro.
First of all, Avapritinib (BLU-285) is a potent and selective exon 17 mutant KIT kinase inhibitor. Therefore, it exhibits in vitro biochemical activity against KIT D816V (IC50=0.27 nM). On one hand, inhibits KIT D816V mutant autophosphorylation in human mast cell leukemia cell lines HMC1.2 and P815 mouse mast cell lines (IC50=4 and 22 nM, respectively). On the other hand, in Kasumi-1 cells (positive AML cell line), it effectively inhibits KIT N822K mutant self-phosphorylation (IC50=40 nM), downstream signaling, and cell proliferation (IC50=75 nM).
Secondly, Avapritinib (BLU-285) is well tolerated in vivo and has antitumor efficacy in vivo. In solid tumor allografts as well as in disseminated disease models, Avapritinib (10 me/kg or 30 mg/kg orally, once daily) significantly inhibited tumor growth.
In summary, Avapritinib (BLU-285) is a highly potent, selective, orally viable KIT and PDGFRA-activating ring mutant kinase inhibitor. It binds to the active conformation of the kinase and effectively targets different mutants of KIT as well as PDGFRA.
References:
[1]. Wu CP, et al. Mol Pharm. 2019 Jul 1;16(7):3040-3052. https://www.ncbi.nlm.nih.gov/pubmed/31117741
[2]. Evans EK, et al. Sci Transl Med. 2017 Nov 1;9(414). pii: eaao1690. https://www.ncbi.nlm.nih.gov/pubmed/29093181
[3]. Erica Evans, et al. Blood 2015 126:568. http://www.bloodjournal.org/content/126/23/568?sso-checked=true