Enhancer of zeste homolog 2 (EZH2) plays an important role in the research of cancer. AS we all know, transcription is important in cell fate decision. Also, dysregulation of transcription is a determinant of cancer phenotype. In addition to genetic alteration, abnormal epigenetic regulation of transcription plays an important role in carcinogenesis and cancer development. However, EZH2 is a member of the family of polycomb group genes (PcGs), which is a group of important epigenetic regulators that repress transcription. Moreover, overexpression of EZH2 has been observed in multiple solid and malignant tumors, including tumors of prostate, breast, kidneys, and lungs, myeloma, and leukemia, and its expression level often correlates with disease progression and poor prognosis. Therefore, we will introduce a selective covalent EZH2 inhibitor-BBDDL2059.
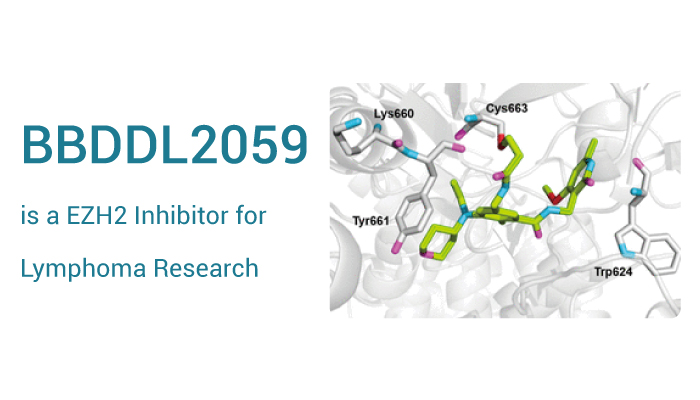
BBDDL2059 is a highly potent and selective covalent inhibitor of EZH2.
BBDDL2059 not only inhibits EZH2 enzymatic activity at sub-nanomolar concentrations (IC50 value of 1.5 nM for EZH2-Y641F), but also achieves low nanomolar potencies in cell growth inhibition.
BBDDL2059 (0-65 nM; 6 days) inhibits cell growth in KARPAS-422 and Pfeiffer cells. In addition, BBDDL2059 (0-1 μM; 48-96 h) inhibits EZH2 enzymatic activity and maintains long-lasting inhibition of EZH2 after washing out.
Besides, BBDDL2059 (3 mg/kg for i.v., 10 mg/kg for p.o.) shows a T1/2 of 0.28 h (i.v.), and oral bioavailability (F%) of 0.05% in rats.
EZH2 has been validated as an attractive therapeutic target for cancer. One of the first-generation inhibitors of EZH2, EZP6438, has received approval from the FDA in 2020. However, the firstgeneration EZH2 inhibitors suffer from high dosage, cofactor S-adenosylmethionine (SAM) competition, and acquired drug resistance. Interestingly, the kinetic assay revealed that BBDDL2059 is noncompetitive with the cofactor SAM.
In a word, BBDDL2059, a covalent inhibition of EZH2, can offer a new opportunity for the development of a promising new generation of EZH2 target research.
Reference:
[1] Zhang Y, et al. J Med Chem. 2023 Jun 8;66(11):7629-7644.
[2] Duan R, et al. J Hematol Oncol. 2020 Jul 28;13(1):104.