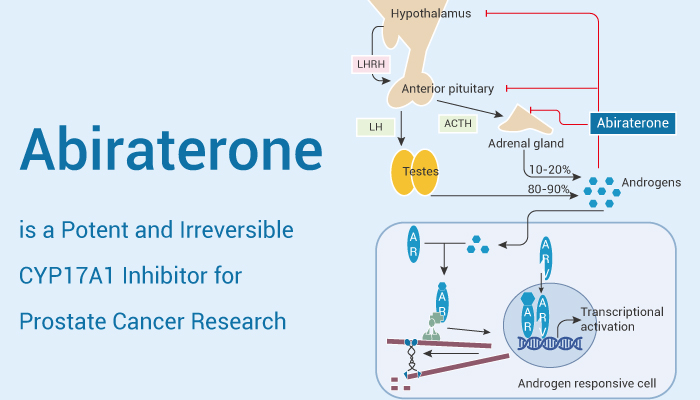
CYP17A1 is a bifunctional hydroxylase that belongs to the cytochrome P450 family. It has 17α-hydroxylase and 17,20-lyase activities and is a key enzyme in the steroidogenic pathway that produces progestins, mineralocorticoids, glucocorticoids, androgens, and estrogens. Androgen receptor (AR), also known as the NR3C4 nuclear receptor, can be activated by binding to any androgen, including testosterone and dihydrotestosterone. Androgen receptors and androgens are involved in the main pathogenesis of prostate cancer. When prostate cancer progresses to castration-resistant prostate (CRPC), androgen synthesis and AR overexpression and/or AR gene amplification and mutations drive cancer development. This process involves the synthesis of steroids.
Here introduce a potent CYP17A1 inhibitor and AR antagonsit, Abiraterone.
Abiraterone, a reported CYP17A1 inhibitor, potently modulates the steroidogenic cascade. It inhibits CYP17A1 17α-hydroxylase and 17,20-lyase activities (IC50: 15 nM and 2.5 nM, respectively), resulting in decreased glucocorticoid and androgen levels and increased mineralocorticoid levels. The 3ß-HSD isoenzyme converts Abiraterone into the Δ4A metabolite (competitive Ki values: 2.1 and 8.8 μM, for recombinant human 3β-HSD1 and 3β-HSD2, respectively). Abiraterone has also been shown to antagonize androgen receptor (AR) activation. Significantly inhibits the proliferation of AR-positive prostate cancer cell lines LNCaP and VCaP. 10 μM Abiraterone completely blocks the synthesis of 5α-diketone and DHT in both cell lines, reducing [3H]-dehydroepiandrosterone (DHEA) consumption and Δ4-androstenedione (AD) accumulation (IC50<1 μM). In xenograft tumors in vivo, Abiraterone slows tumor growth. After intravenous injection (5 mg/kg), the clearance (Cl) and volume of distribution (Vd) are 31.2 mL/min/kg and 1.97 L/kg, respectively.
In summary, Abiraterone is a potent CYP17A1 inhibitor and androgen receptor antagonist, which effectively inhibits the development of prostate cancer.
References:
[1] Fragni M, et al. Naunyn Schmiedebergs Arch Pharmacol. 2019 Jun;392(6):729-742.
[2]. Stein MN, et al. Asian J Androl. 2014 May-Jun;16(3):387-400.
[3]. Li R, et al. Clin Cancer Res. 2012 Jul 1;18(13):3571-9.
[4]. Kumar SV, et al. Biomed Chromatogr. 2013 Feb;27(2):203-7.