Tyrosine kinases are a family of enzymes, which catalyzes phosphorylation of select tyrosine residues in target proteins, using ATP. This covalent post-translational modification is a pivotal component of normal cellular communication and maintenance of homeostasis. Tyrosine kinases are implicated in several steps of neoplastic development and progression. Tyrosine kinases are important mediators of the signaling cascade, determining key roles in diverse biological processes like growth, differentiation, metabolism and apoptosis in response to external and internal stimuli.
Receptor tyrosine kinases (RTKs) are the high-affinity cell surface receptors for many polypeptide growth factors, cytokines, and hormones. Though the activity of tyrosine kinases is tightly regulated in normal cells, they may acquire transforming functions due to mutation(s), overexpression and autocrine paracrine stimulation, leading to malignancy. Thus, RTKs not only are the key regulators of normal cellular processes, but also to have a critical role in the development and progression of many types of cancer. Mutations in RTKs lead to activation of a series of signalling cascades which have numerous effects on protein expression. RTKs are part of the larger family of protein tyrosine kinases, encompassing the RTK proteins which contain a transmembrane domain, as well as the non-RTKs which do not possess transmembrane domains.
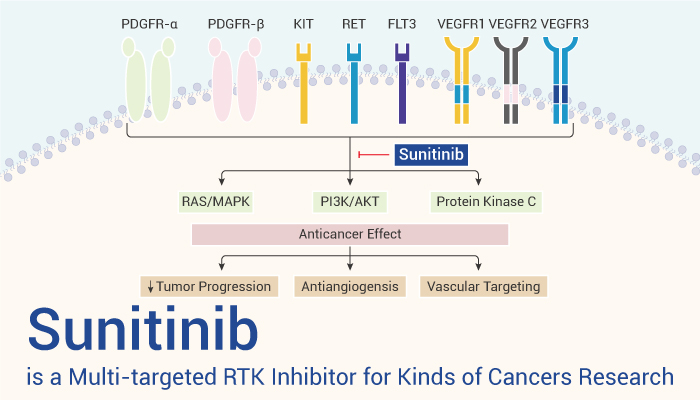
Sunitinib (SU 11248) is a multi-targeted receptor tyrosine kinase inhibitor
Sunitinib inhibits cellular signalling by targeting multiple RTKs. These include all receptors for platelet-derived growth factor (PDGF-Rs) and vascular endothelial growth factor receptors (VEGFRs), which play a role in both tumor angiogenesis and tumor cell proliferation. The simultaneous inhibition of these targets therefore reduces tumor vascularization and triggers cancer cell apoptosis and thus results in tumor shrinkage. Besides, Sunitinib is also an ATP-competitive inhibitor, effectively inhibits autophosphorylation of Ire1α by inhibiting autophosphorylation and consequent RNase activation. Sunitinib has very good oral bioavailability, is highly efficacious in a number of preclinical tumor models, and exhibits good tolerance at efficacious doses. For example, Sunitinib inhibits the growth of established SF763T and Colo205 tumor xenografts in athymic mice.
All in all, Sunitinib is effective in renal cell carcinoma (RCC) and imatinib-resistant gastrointestinal stromal tumor (GIST).
References:
[1] Du Z, et, al. Mol Cancer. 2018 Feb 19;17(1):58.
[2] Sun L, et, al. J Med Chem. 2003 Mar 27;46(7):1116-9.