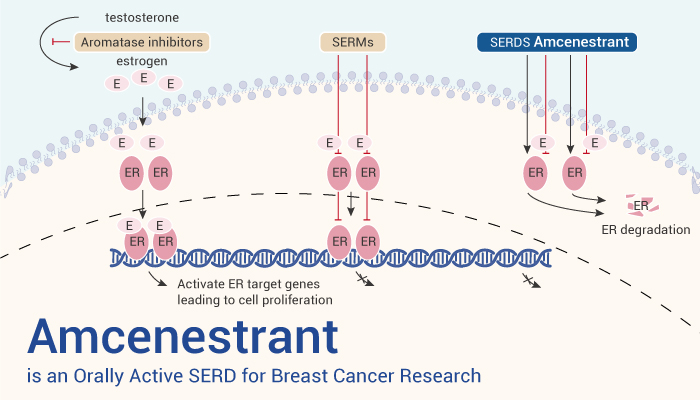
Selective estrogen receptor degrading or downregulating (SERD) is a drug that binds to estrogen receptors (ER). Specifically, ER regulates various complex physiological processes in humans. Abnormal ER signaling may lead to various diseases, including reproductive system related diseases, cardiovascular diseases, neurodegenerative diseases, and skin melanoma. Meanwhile, SERD can induce effective inhibition of ER signaling. For example, fluvastatin, combined with ER α combining induced conformational changes not only antagonizes ER α Function, and more effectively inhibit ER through proteasome mediated degradation α Signal transduction. Nonetheless, SERD and total receptor antagonists provide important therapeutic options for hormone receptor (HR) positive breast cancer. Now, we will introduce an orally active, nonsteroidal and selective estrogen receptor degrader (SERD), Amcenestrant.
Amcenestrant is an Orally Active SERD for Breast Cancer Research.
First of all, Amcenestrant (SAR439859) has ER degrading activity with an EC50 of 0.2 nM for ERα degradation. Importantly, Amcenestrant demonstrates robust antitumor efficacy and limited cross-resistance in ER+ breast cancer.
In the second place, Amcenestrant induces strong antitumor activity against a variety of BC cell lines and patient-derived xenografts, including models that harbor ERα mutations. In particular, Amcenestrant with 2.5-25 mg/kg twice daily for 30 days by orally exhibits substantial tumor-growth inhibition. Obviously, Amcenestrant displays tumor regression at the dose of 25 mg/kg/BID.
Last but not the least, Amcenestrant shows a low to moderate clearance in the three animal species tested (0.03-1.92 L/h•kg). Interestingly, Amcenestrant has low to moderate volume of distribution (Vss=0.5-6.1 L/kg), and good bioavailability (54-76%) across species. It is noticed that T1/2 was variable across species (1.98 h in mouse, 4.13 h in rat and 9.80 h in dog).
All in all, Amcenestrant is an orally active SERD for breast cancer research.
References:
[1] El-Ahmad Y, et al. J Med Chem. 2020 Jan 23;63(2):512-528.