Estrogen is a critical steroid in human physiology, the female sex hormone, exerts anti-inflammatory and anti-atherogenic effects. Traditionally, estrogen effects were believed to be largely mediated through the classical estrogen receptors (ERs). However, there is increasing evidence that G-protein coupled receptor 30 (GPR30), a novel estrogen receptor, can mediate many estrogenic effects on the vasculature.
Tumor necrosis factor (TNF), a pro-inflammatory cytokine that play an important role in atherogenesis and many other inflammatory conditions, can induce inflammatory changes. We found that GPR30 was located predominantly in the endothelial cell nuclei. Treatment with the selective GPR30 agonist G-1 partially attenuated the TNF induced upregulation of pro-inflammatory proteins.
G-1 is a nonsteroidal, high-affinity and selective agonist of GPR30.
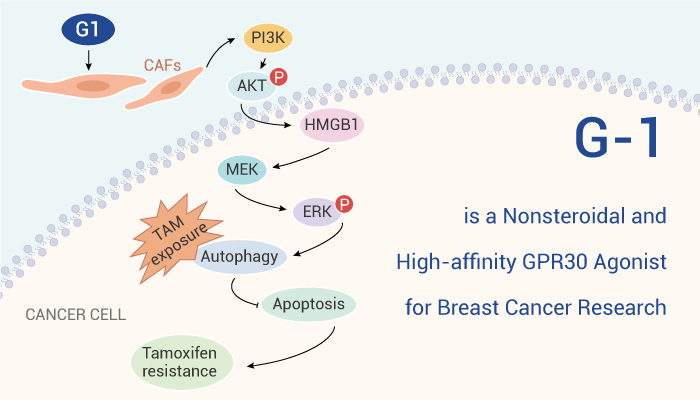
Treatment with G-1 significantly decreases A549 cells proliferation. G-1 also increases apoptosis of A549 cells, consistent with its antiproliferative effect. Besides, G-1 induces a cell cycle arrest in the G2 phase in H295R cells. The presence of G-1 increases Bax expression while decreases Bcl-2. Similar to estrogen, the specific GPR30 agonist, G-1, significantly increases the amplitude of excitatory postsynaptic currents. Furthermore, G-1 exerts neuroprotective effects against NMDA-induced oxidative toxicity in vitro and inhibits osteoporosis in ovariectomized (OVX) rats.
All in all, G-1 is a nonsteroidal, high-affinity and selective GPR30 agonist that exhibits anti-inflammatory, anti-tumor and neuroprotective activities.
References:
[1] Chakrabarti S, et, al. PLoS One. 2012;7(12):e52357.
[2] Cheng Q, et, al. Biosci Rep. 2016 Aug 31;36(4):e00373.