Triple-negative breast cancer (TNBC) is an aggressive subtype of breast cancer. It accounts for approximately 15%–20% of newly diagnosed cases with poor clinical outcomes.
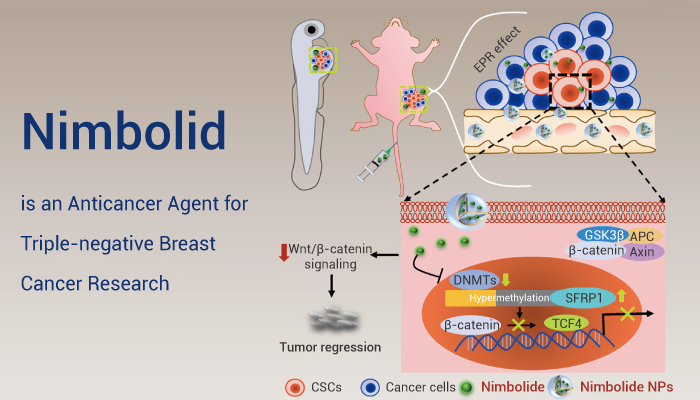
Recently, phytochemicals have garnered significant attention as anticancer agents. Nimbolide (Nim) is a limonoid triterpene derived from the leaves of Azadirachta indica. It has potent anticancer effects by regulating oxidative stress and inhibiting cell proliferation, EMT, metastasis, and angiogenesis in several cancers. In this context, scientists investigate Nimbolide. At first, formulate Nim-encapsulated poly(lactic-co-glycolic acid) (PLGA) nanoparticles (Nim NPs). And they evaluate the anti-cancer stem cell (CSC) effects in vitro and in vivo.
In vitro studies suggested that Nim NPs significantly inhibited several inherent characteristics of BCSCs, such as stemness, self-renewability, chemoresistance, epithelial-to-mesenchymal transition (EMT), and migration in comparison to native Nim. Next, the mechanism behind the anti-CSC effect of Nim was explored. Mechanistically, they found that Nim epigenetically restores tumor suppressor gene secreted frizzled-related protein 1 (SFRP1) expression by downregulating DNA methyltransferases (DNMTs), leading to Wnt/β-catenin signaling inhibition. Further, in vivo results demonstrated that Nim NPs showed enhanced anti-tumor and anti-metastatic effects compared to native Nim in two preclinical models without any systemic toxicity. Overall, Nim-based phytonanomedicine can inhibit BCSCs by epigenetic reprogramming of the DNMTs-SFRP1-Wnt/β-catenin signaling axis.
Nimbolide is a triterpene compound with anticancer and antiproliferative activity.
Other studies find that Nimbolide induces tumor cell apoptosis by inhibiting NF-κB and CDK4/CDK6 kinase. It suppresses the Wnt, PI3K-Akt, MAPK and JAK-STAT signaling pathways.
In Vitro: Firstly, Nimbolide (2 μM) blocks growth factor-induced phosphorylation of Akt, ERK1/2, and STAT3 in U87EGFRvIII cells. It (2-6 μM) inhibits the IGF-I-mediated breast cancer cell proliferation in MCF-7/MDA-MB-231 cells. Secondly, Nimbolide (2-6 μM) inhibits proliferation and causes G0/G1-phase arrest through repression of cyclins (A1/B/C/D1/E1) in MCF-7/MDA-MB-231 cells. It (0-6 μM) also increases the expression of DUSP4, increases E-cadherin, Snail and MMP-3 expression. Thirdly, Nimbolide (0-6 μM, 12 h) suppresses invasion and migration of NSCLC cells. Meanwhile, Nimbolide (10 μM, 0-12 h) inhibits NF-κB activation in HCT-116/HT-29/Caco-2 cells by a time-dependent manner.
In Vivo: Nimbolide (10 mg/kg, i.v., daily, 7 days) inhibits glioblastoma multiforme tumor growth in U87EGFRvⅢ xenografted mouse model. In addition, Nimbolide (5 mg/kg, 20 mg/kg; i.p.; daily, 10 days), inhibits tumor growth in HCT-116-derived xenograft mouse, reduces the expression of proteins involved in invasion, metastasis, and angiogenesis (MMP-9, CXCR4, ICAM-1, and VEGF).
Reference:
[1] Mohapatra P, et al. Mol Ther Nucleic Acids. 2023 Sep 9;34:102031.
[2] Lin H, et al. 2017 Aug;92:340-346.