CDK4/6 is a Ser/Thr protein kinase that is active only in the G1-S phase. It is controlled by the regulatory subunit D-type cyclin and the CDK inhibitor p16INK4a; involved in the phosphorylation of the retinoblastoma gene product (Rb). Here we introduce an orally effective CDK4/6 inhibitor, Trilaciclib (G1T28). It exhibits IC50 values of 1 nM and 4 nM for CDK4 and CDK6, respectively.
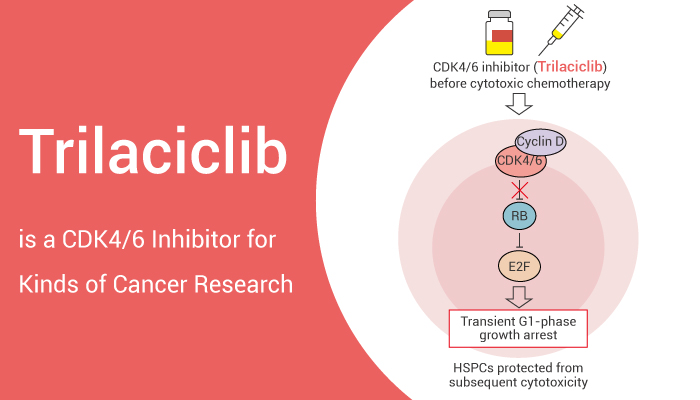
Trilaciclib is a CDK4/6 inhibitor to suppress tumors and chemotherapy damage.
In chemotherapy, Trilaciclib may alleviate some of the harmful side effects associated with chemotherapy. However, it transiently and reversibly modulates the proliferation of mouse and canine bone marrow hematopoietic stem and progenitor cells (HSPCs), provides protection against multiple lineages, and mitigates chemotherapy-induced hematological toxicity. Moreover, it inhibits phosphorylation of RB and induces CDK4/6-dependent cell cycle arrest at G1. Trilaciclib protects RB-competent cells from chemotherapy damage in vitro and in vivo in a γ-H2A.X (γH2AX) assessment and reduces apoptosis by downregulating caspase 3/7 activation.
As for in vitro study, on the once hand, Trilaciclib (10-1000 nM; 24 h) reversibly modulates the proliferation of mouse and canine bone marrow hematopoietic stem and progenitor cells in vitro. It (10-1000 nM; 24 h) arrests the cell cycle in the G1 phase of CDK4/6-dependent cells with an EC50 of 30 nM for HS68. And, it (300 nM; 16 or 48 h) protects CDK4/6-dependent cells (HS68, WM2664) from chemotherapy-induced damage and attenuates chemotherapy-induced apoptosis.
In in vivo studies, on another hand, trilaciclib (50-150 mg/kg; oral; single dose) protected mouse bone marrow cells from chemotherapy-induced apoptosis and dose-dependently attenuated chemotherapy-induced myelosuppression in vivo. Trilaciclib at 150 mg/kg reduces HSPC damage induced by 5FU (HY-90006) (150 mg/kg; ip) chemotherapy, thereby accelerating recovery of blood counts after chemotherapy.
In summary, Trilaciclib is an orally effective CDK4/6 inhibitor with anti-tumor activity, and can weaken the toxic effects of chemotherapy on cells.
Reference:
[1] Bisi JE, et al. Mol Cancer Ther. 2016 May;15(5):783-93.