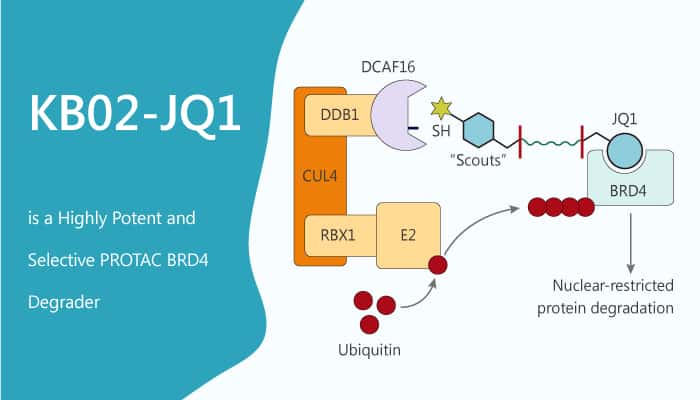
DDB1-CUL4-associated protein 16 (DCAF16) is a 216 aa protein that is highly conserved across mammals, but absent from rodents. The DCAF16 protein has eight cysteine residues, including a cluster of four cysteines between amino acids 173-179. Especially, DCAF16 is a substrate recognition component of CUL4-DDB1 E3 ubiquitin ligases. DCAF16 acts as a target of electrophilic PROTACs that promote the nuclear-restricted degradation of proteins.
DCAF16 acts as the KB02 compound-binding factor. KB02-JQ1 is a bifunctional PROTAC consists of KB02 and JQ1. JQ1 is a highly potent, and selective bromodomain-containing protein 4 (BRD4) inhibitor.KB02-JQ1 induces the destruction of BRD4, but not its close family members BRD2 and BRD3. KB02-JQ1 promotes the degradation of BRD4 in HEK293T cells in a concentration-dependent manner,
In this study, Concentration-dependent degradation of endogenous BRD4 in HEK293T cells following treatment with KB02-JQ1 (5, 10, 20, 40 μM) for 24 h. The two separate components of KB02-JQ1, KB02, and JQ1 do not degrade BRD4 in cells. FKBP12-directed bifunctional compound KB02-SLF also does not degrade BRD4 in cells. KB02-JQ1 degrades BRD4 by sub-stoichiometric modification of DCAF16. MG132 or MLN4924 block this degradation effect. HEK293T cells preincubate with 10 µM MG132 or 1 µM MLN4924 for 4 h, followed by 20 h of treatment with 20 µM KB02-JQ1 and 10 µM MG132 or 1 µM MLN4924. The proteasome inhibitor MG132 and neddylation inhibitor MLN4924 block KB02-JQ1-mediated BRD4 degradation.
All in all, ligand-dependent protein degradation has emerged as a compelling strategy to pharmacologically control the protein content of cells. KB02-JQ1 is an electrophilic PROTAC that degrades nuclear proteins by engaging DCAF16.