CDKs (Cyclin-dependent kinases) are the families of protein kinases first discovered for their role in regulating the cell cycle. By definition, a CDK binds a regulatory protein called a cyclin. Without cyclin, CDK has little kinase activity; only the cyclin-CDK complex is an active kinase. The four major mechanisms of CDK regulation are cyclin binding, CAK phosphorylation, regulatory inhibitory phosphorylation, and binding of CDK inhibitory subunits (CKIs). In addition, CDK4/6 regulates cell cycle progression by phosphorylating and inactivating the tumor suppressor retinoblastoma protein (RB). Recently, hetero-bifunctional small molecules (PROTACs) that designed to achieve selective degradation of target proteins, targeting many target kinases, including CDK4/6.
XY028-140 (MS140) is a potent and selective PROTAC-based CDK4/CDK6 kinase degrader. XY028-140 specifically inhibits RB-E2F signaling and reduces CDK4 and CDK6 protein levels in a dose- and time-dependent manner in vitro. In addition, XY028-140 can degrade its targets by linking them with ubiquitin-proteasome mechanism. Further, the degradation of CDK4/6 protein by XY028-140 is specifically mediated by CRBN. Therefore, XY028-140 effectively and selectively inhibits and degrades CDK4/6 kinase by targeting CDK4/6 kinase in CDK4/6i-S (CDK4/6 inhibitor-sensitive) tumor cells to the CRL4-CRBN-E3 ubiquitin complex. Compared with CDK6 wild-type cells, XY028-140 treatment of cells expressing wild-type or mutation-activated CDK6 resulted in a more effective degradation of CDK6 (S178P).
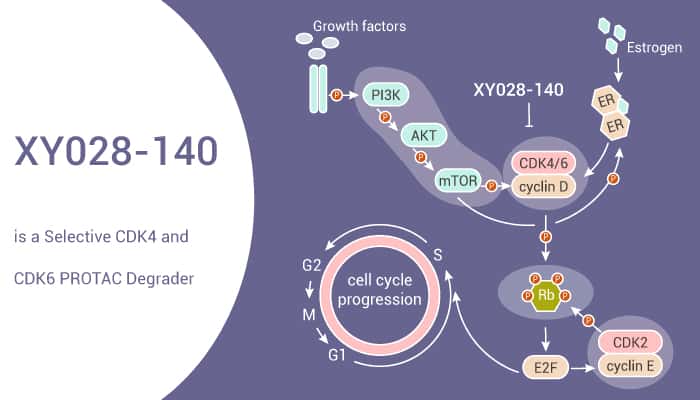
To sum up, XY028-140 is a potent and selective CDK4/6 degrader, which can inhibit RB-E2F signaling and reduce CDK4 and CDK6 protein levels.
References:
[1] Xuewei Wu, et al. Nat Cancer 2, 429-443 (2021).