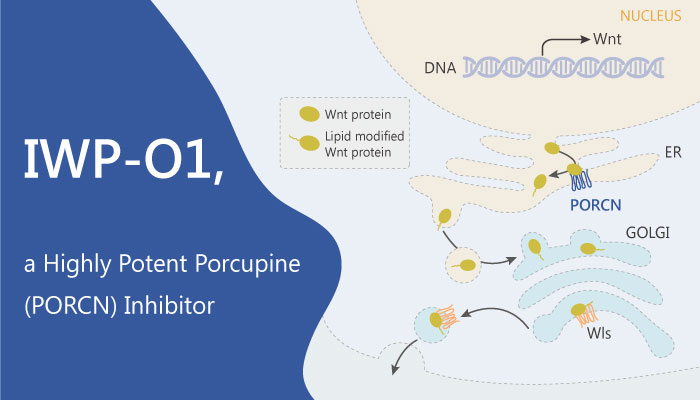
Secreted Wnt proteins play essential roles in embryonic development and adult tissue homeostasis. And the acyltransferase Porcupine (Porcn) is essential for the secretion of Wnt proteins which contribute to embryonic development, tissue regeneration, and tumorigenesis. In previous studies, Lin You et al describe herein the development of a new class of small-molecule Porcn inhibitors that is highly active in a cultured cell reporter assay of Wnt signaling. A recent study from Lin You discovered and described a tnovel Porcn inhibitor IWP-O1.
The authors have previously identified four classes of small-molecule Porcn inhibitors from a high-throughput screen (HTS). A close examination of their structures led to the identification of a common structural feature wherein an aryl amide is attached to a heteroaromatic ring through a heteroatom.
IWP-O1 is a highly potent Porcupine (Porcn) inhibitor, with an EC50 of 80 pM in L-Wnt-STF cells.
Dishevelled (Dvl) phosphorylation is associated with both β-catenin dependent and independent Wnt signaling pathways. IWP-O1 effectively suppressed the phosphorylation of Dvl2/3 in HeLa cells. At the same time, the phosphorylation of low density lipoprotein receptor-related protein 6 (LRP6). It is a hallmark of the Wnt/β-catenin pathway activity, was also suppressed by the chemical treatment.
Furthermore, IWP-O1, with a 1,2,3-triazole core, is much more stable in murine liver S9 fractions and plasma than other compounds found. Particularly, IWP-O1 suppresses Wnt signaling in L-Wnt-STF cells with an EC50 value of 80 pM, 2.5 times more active than the investigational drug LGK974. With significantly improved metabolic stability, IWP-O1 is more suitable for model studies in mice.