G protein-coupled receptors (GPCRs) exhibit a spectrum of functional behaviors in response to natural and synthetic ligands. Researchers generated the human β2 adrenergic receptor (β2AR) that exhibits G protein-like behavior. The active G protein-coupled state of the β2AR exhibits characteristic functional properties. Agonists promote Gs binding to the β2AR and G protein binding to the receptor increases agonist affinity.
Researchers identified a camelid antibody fragment that exhibits G protein-like behavior towards the β2AR. β2 adrenergic receptor (β2-AR) is the most common adrenergic receptor subtype expressed in various types of lung cells, including airway epithelial cells. Moreover, β2-AR is a well-known GPCR and has many potential sites of phosphorylation. A variety of β2-adrenoceptor agonists are currently under development with the hopes of achieving once-daily dosing. β2-adrenoceptor agonists are an important pharmacological approach. This approach induces bronchodilation in patients suffering from the chronic obstructive pulmonary disease (COPD) and asthma. β2-adrenoceptor agonists increase airway hyperresponsiveness. Furthermore, β2-adrenoceptor agonists remain effective bronchodilators available for the immediate relief of asthma symptoms.
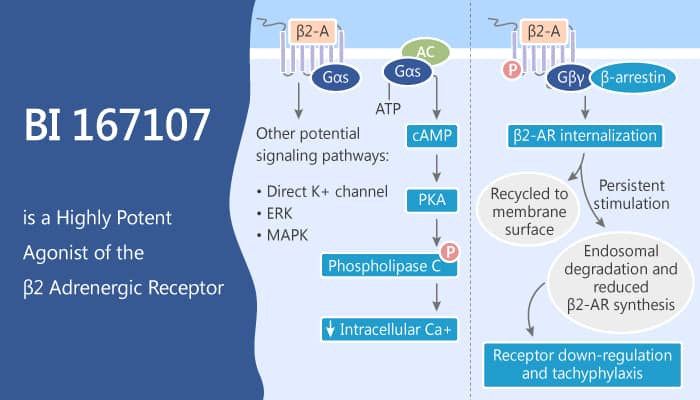
β2-AR activation normally leads to increased intracellular cAMP levels. As a result, cAMP production normally leads to activation of the catalytic subunit of PKA. The effect phosphorylates cAMP-responsive element-binding protein (CREB), allowing it to bind to CRE sites. Especially, BI-167107 is a full agonist that binds to the β2AR with a dissociation constant Kd of 84 pM. In particular, BI-167107 induces a larger change in the fluorescence intensity and λmax of bimane bound to C265 than does the agonist isoproterenol.
All in all, BI-167107 is a full agonist that binds to the β2AR with high affinity.