Ubiquitin-like with PHD and RING finger domains 1 (UHRF1) is a potent oncogenic protein. Its overexpression in various solid and blood tumors. Moreover, UHRF1 has an association with reduced expression of tumor suppressor genes, including BRCA1 and PPARγ. UHRF1 contains five functional domains, and each domain could independently carry out epigenetic functions of UHRF1. SRA domain is unique to UHRF1. It directly recognizes hemimethylated DNA and recruits DNA methyltransferase 1 (DNMT1) to chromatin for DNA methylation maintenance. In addition, its TTD functions as a histone reader to bind the transcription repressive marker of H3K9me2 or H3K9me3. Therefore, TTD recognition of the methylated H3K9 mark is essential for the recruitment of DNMT1 to daughter strands during DNA replication. Thus, the TTD is an exciting target. NV03 is a potent and selective antagonist of UHRF1-H3K9me3 interaction by binding to the UHRF1 Tandem Tudor Domain (Kd=2.4 μM).
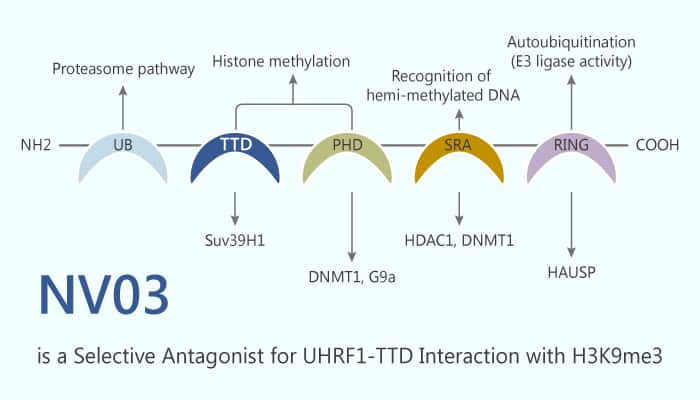
Tumorigenesis is associated with abnormal DNA methylation patterns. Meanwhile, it affects the expression of tumor suppressor genes. In breast cancer, UHRF1 overexpression induces the hypermethylation and inhibition of BRCA1 by forming an inhibitory transcriptional complex comprising HDAC1, DNMT1, and G9a. Consistently, hepatocellular carcinoma (HCC) highly expresses UHRF1, leading to delocalization of DNMT1, induces DNA hypomethylation, and Tp53- mediated senescence. Therefore, antagonism of UHRF1 emerges as an attractive therapeutic strategy for the treatment of a variety of cancers associated with UHRF1 overexpression.
Overall, NV03 has anticancer activity and is a potent antagonist for UHRF1-TTD interaction with H3K9me3. Furthermore, these studies provide a solid starting point for the future development of chemical probes to study the cellular functions of the UHRF1 histone reader tudor domain and the therapeutic potential of its inhibition.
Reference:
Senisterra G, et al. SLAS Discov. 2018 Oct;23(9):930-940.