Genetic alterations that disable G1/S checkpoint control and loss of this checkpoint often happen in human cancer. The mechanisms contribute to cancer generation by permitting inappropriate proliferation and distorting fate-driven cell cycle exit. Therefore, the active compounds activate the G1/S checkpoint. And they may represent a broadly applicable and clinically effective strategy for the treatment of cancer. A study from Simon R. Stockwell discovered and identified CCT020312 as the G1/S checkpoint activator.
During the study, the authors treated HT29 cells with CCT020312 for 24 hours. As a result, CCT020312 revealed a concentration-dependent loss of P-S608-pRB signal, with a linear response between 1.8 and 6.1 μM. Flow-cytometry of HT29 cells exposed to 10 μM CCT020312 revealed an increased number of cells in the G1 phase of the cell cycle at 16 and 24 hours as well as effective reduction of DNA synthesis. Additionally, CCT020312 reduced the amount of the G1/S cyclins D1, D2, E and A as well as the CDK catalytic subunit CDK2 and increased the level of the CDK inhibitor p27KIP1 present in HT29 cells.
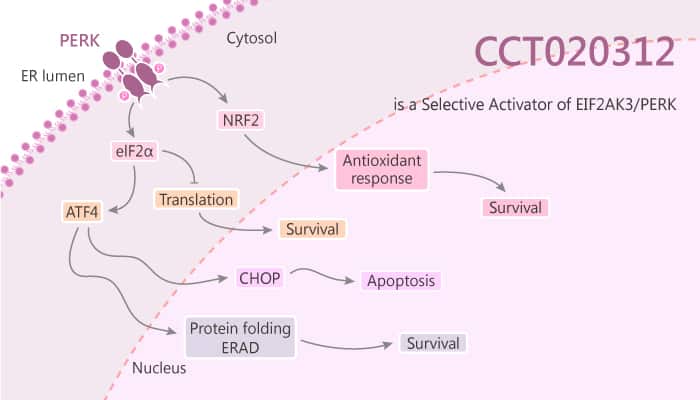
Interestingly, previous studies have indicated a link between ER stress-mediated EIF2AK3/PERK signalling and the cellular sensitivity to multiple anticancer agents, including taxanes. As a result, CCT020312 with the concentration of 2.5 μM resulted in a clear augmentation of the paclitaxel-induced growth inhibition in U2OS cells.
In the study, the authors also hypothesised that CCT020312 might function via inhibiting HSP90 or PI3 kinase or by regulating EIF2A phosphorylation. CCT020312 acts as an effector of Ser51 EIF2A phosphorylation.