The bromodomain-containing protein BRD9, a recently identified mSWI/SNF subunit, is a novel complex configuration distinct from BAF and PBAF. The mSWI/SNF (BAF) complex comprises at least 15 subunits of ~2 MDa in size. The BAF complex has an autonomous capacity to specifically regulate gene expression patterns in a cell lineage restricted manner. Moreover,The BAF complex is dynamic and exhibits considerable plurality.
BRD9 defines a novel mammalian SWI/SNF (BAF) complex configuration which supports proliferation in acute myeloid leukemia (AML). AML is a cancer of the myeloid line of blood cells.
BRD9 is a bromodomain-containing protein that forms a small sub-branch of the bromodomain family tree. Human BRD9 contains a single bromodomain and has five isoforms that are produced by alternative splicing. BRD9 is a subunit of the human BAF (SWI/SNF) nucleosome remodeling complex. BRD9 has emerged as an attractive therapeutic target in cancer. Moreover, Small-molecule inhibitors of BRD4 established the feasibility of inhibiting acetyl-lysine recognition domains (bromodomains).
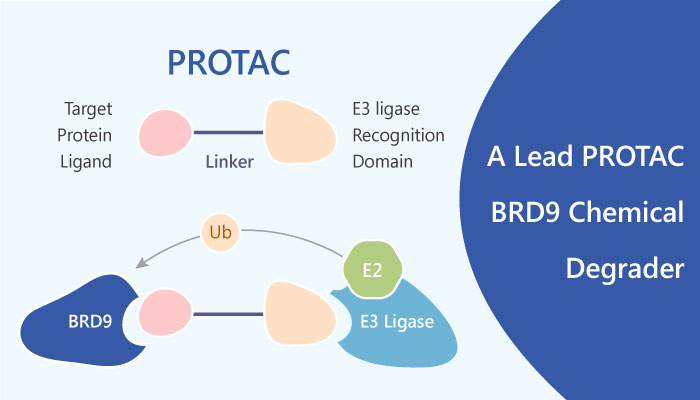
Recently, Remillard D, et al reported a strategy to direct protein-specific degradation using bifunctional molecules to recruit the cereblon (CRBN) ubiquitin ligase complex to non-physiologic protein substrates, providing an all-chemical solution to prior efforts using peptides to bridge E3 ligases and ligand targets (PROTACs). Degraders of BRD9 exhibit markedly enhanced potency compared to parental ligands. Hopefully, PROTAC BRD9 Degrader-1 is a lead PROTAC BRD9 chemical degrader, which can be used as a selective probe useful for the study of BAF complex biology.