Greatwall kinase (GWL) is a critical regulator of mitosis and meiosis. It regulates PP2A-B55 activity by phosphorylating two proteins, Arpp19 and ENSA. This phosphorylation turns these proteins into potent inhibitors of PP2A-B55, thereby promoting a correct timing and progression of mitosis. When over-expressed in breast cancer, GWL induces oncogenic properties such as transformation and invasiveness. Conversely, down-regulation of GWL selectively sensitises tumour cells to chemotherapy. Ocasio CA, et al, have developed a first generation Greatwall kinase inhibitor GKI-1 to study human GWL as a target for drug discovery.
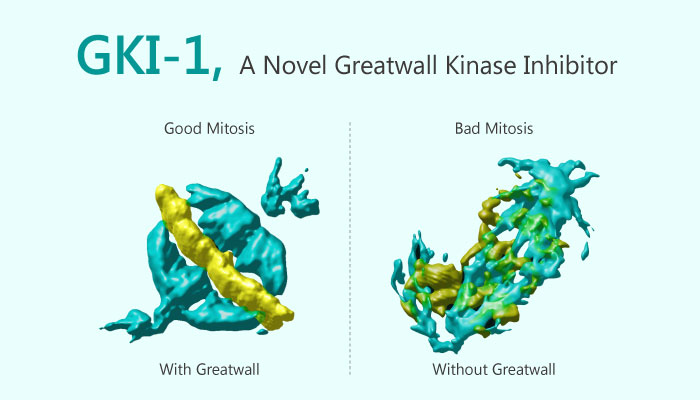
GKI-1 inhibits full-length human GWL . It had no observable inhibitory activity towards CDK2 at concentrations up to 100 μM. It robustly inhibited ROCK1 with an IC50 of ~11 μM, but only weakly affected PKA. At the 25 μM concentration tested, GKI-1 clearly has several off-target interactions, but they are particularly focussed around the AGC kinase family. Treatment of HeLa cells with GKI-1 reduces ENSA/ARPP19 phosphorylation levels. It also results in a decrease in mitotic events, mitotic arrest/cell death and cytokinesis failure.
Despite the in vitro off-target range of GKI-1, it appears to cause cellular phenotypes that are surprisingly similar to the effects caused by GWL siRNA treatment. However, GWL depletion causes a more pronounced failure in cytokinesis that are rarely observed in GKI-1 treated cells. This difference could be due to the incomplete inactivation of the kinase by the compound, or could point to a kinase-independent function of GWL. Clearly, further work will be required to precisely define on- and off-target effects of GKI-1. It will be a useful starting point for the development of more potent and selective GWL inhibitors.