Chronic myeloid leukemia (CML) is a white blood cell disorder, accounting for about 15% of adult leukemias. The critical driving force of pathogenesis of CML identified is BCR gene and ABL kinase gene fusion. It results in the constitutive activation of ABL kinase. And the activation is essential for the subsequent activation of the mitogenic signaling pathways, suppression of the apoptosis as well as the enhanced adhesive capability to stromal cells for the proliferation.
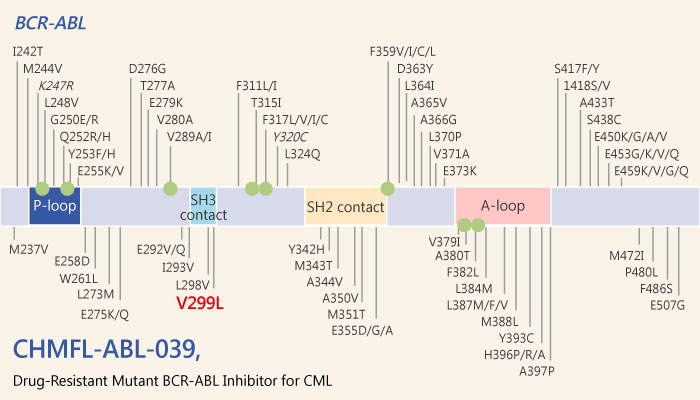
Thus, inhibition of the BCR-ABL kinase activity is an effective approach to control the CML. Imatinib is the first BCR-ABL kinase inhibitor. Moreover, it achieves great success in the clinic. However, it didn’t show any efficacy in a variety of drug resistance mutants, such as F317L, H369P, Y253H, and V299L.
Additionally, CHMFL-ABL-039, is a highly potent and selective type II native and drug-resistant V299L mutant BCR-ABL inhibitor with IC50 of 27.9 nM. And it also exhibits a binding Kd of 228 nM to ABL V299L mutant.
In vitro, CHMFL-ABL-039 is 6-10 fold more sensitive to BCRABL driven cancer cell lines compared Imatinib. HL-60, MOLM-14, MV4-11 and U937 cells display a great selectivity window comparing to the BCR-ABL driven cell lines. CHMFL-ABL-039 exhibits a similar range of anti-proliferative effect against CD34+ cells. Consequently, it indicates no general cytotoxicity. Meanwhile, CHMFL-ABL-039 can dose dependently inhibit the ABL Y245 phosphorylation and the subsequent downstream signaling mediators, such as pSTAT5 Y694, pERK T202/204 in K562, KU812, MEG-01, and BaF3-BCR-ABL-V299L.
In vivo, CHMFL-ABL-039 do not exhibit any apparent general toxicity and do not affect the mouse weight. CHMFL-ABL-039 can dose dependently suppress the tumor progression for both models at either dosage. In the Imatinib insensitive BaF3-BCR-ABL-V299L mutant cells mediated xenograft model, 25 mg/kg dosage of CHMFL-ABL-039 displays similar efficacy as 100 mg/kg.
In summary, CHMFL-ABL-039 ,a highly potent and selective BCR-ABL V299L mutant kinase inhibitor, may reserve as a good pharmacological tool to study V299L associated tumorigenesis.
Receference:
Wu J, et al. Cancer Biol Ther. 2019;20(6):877-885.