Autophagy is an evolutionarily conserved and stress-responsive catabolic process. Particularly, cellular components and damaged organelles are sequestered within autophagosomes for lysosomal degradation. Autophagy is a multi-step process. Autophagosome biogenesis requires 2 ubiquitin-like conjugation systems: the Atg12-Atg5 and the Atg8–phosphatidylethanolamine (PE) systems. Importantly, the cysteine protease ATG4B is a key component of the autophagy machinery. ATG4B acts to proteolytically prime and recycle its substrate MAP1LC3B. ATG4B is an autophagy protein of interest as a potential therapeutic target due to its roles in autophagosome formation and maturation. Human ATG4B plays crucial roles in cancer development.
In this study, Yuanyuan Fu, et al integrated in silico screening and in vitro assays to discover a potent ATG4B inhibitor S130. Especially, S130 has a high potent and selective inhibition for ATG4B with an IC50 of 3.24 μM. Suppression of ATG4B by S130 mainly affects the turnover of auto-lysosomes. Moreover, S130 results in the accumulation of undigested auto-lysosomes. S130 is effective in arresting the growth of colorectal cancer cells. Besides, S130 has significant cytotoxic effects on adherent cells, such as HeLa (IC50=16.1 μM) and HCT116 (IC50=9.0 μM), as well as on cells in suspension, such as HL60 (IC50=4.7 μM). S130 significantly attenuates the growth of xenografted colorectal cancer cells, especially when combines with caloric restriction. The anti-tumor effect of S130 might be due to the suppression of autophagy, activation of apoptosis, and increased susceptibility to stress.
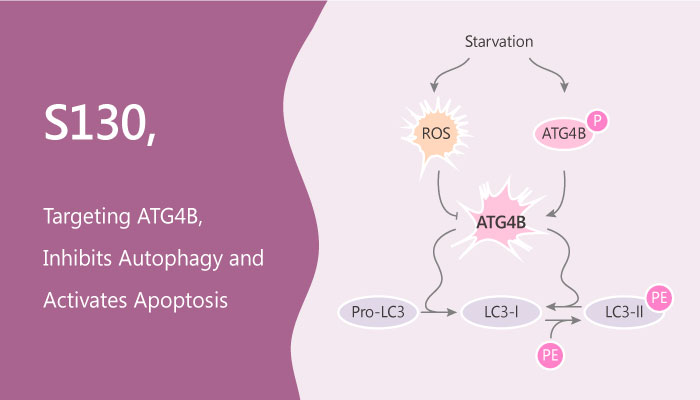
Taken together, S130 might be a promising pharmacological ATG4B inhibitor for autophagy inhibition and tumor suppression.