Heat shock protein 70 (Hsp70/HSPA1A) is a molecular chaperone. It plays important roles in protein homeostasis and cell survival. The expression of Hsp70 strongly correlates with tumor grade, metastasis, and poor prognosis. Thus, it plays a special and broad role in cancer. Hsp70 collaborates with a wide range of co-chaperones, including a family of proteins that contain conserved Bag domains. These Bag domains bind to Hsp70 and help guide its chaperone functions. However, Bag3 is selectively up-regulated in response to stress. Hsp70 co-elevates with its expression in many tumor types. Even more importantly, Bag3 has been shown to collaborate with Hsp70 in regulating cancer development through multiple pathways, including the cell cycle and suppression of oncogene-induced senescence.
JG-98, an allosteric inhibitor, binds tightly to a conserved site on Hsp70 and weakens the Hsp70-Bag3 interaction in vitro and in cells. Therefore, JG-98 strongly inhibits the interactions between Hsp70 and Bag3, with an IC50 value of 1.6 μM, Consistent with the Bag3 results, JG-98 is a good inhibitor of the Hsp70-Bag1 and Hsp70-Bag2 complexes, with IC50 values of 0.6 and 1.2 μM, respectively.
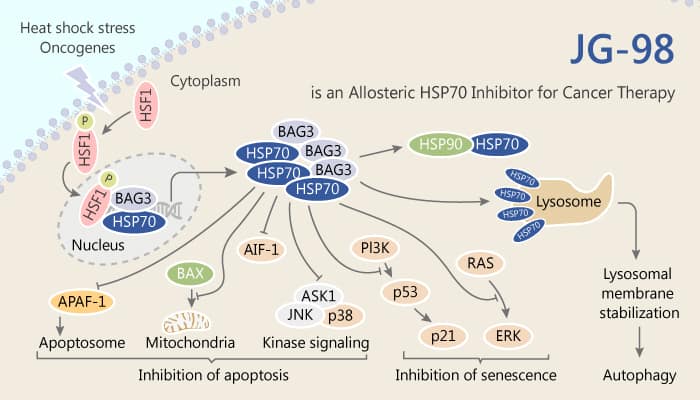
In vitro, JG-98 inhibits the breast cancer cell lines MDA-MB-231 and MCF-7 with EC50 values of 0.4 and 0.7 μM, respectively. Meanwhile, it has antiproliferative activity across a range of cell lines. JG-98 activates both apoptotic mediators (cleavage of caspase-3 and PARP) in MDA-MB-231 cells. In addition, JG-98 destabilizes FoxM1 and relieves suppression of downstream effectors, including p21 and p27.
In vivo, JG-98 do not decreases significant weight loss in treated NSG mice. Moreover, JG-98 suppressed tumor growth in xenograft models bearing MCF7 and HeLa cells.
All in all, JG-98 is a potential anticancer agent and Hsp70-Bag3 interaction may be a promising target for further exploration.
Reference:
Li X, et al. Mol Cancer Ther. 2015 Mar;14(3):642-8.