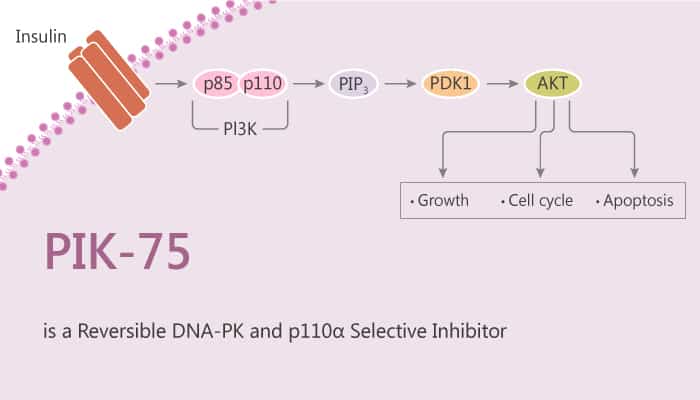
PI3Ks catalyze the phosphorylation of the D-3 position of the inositol headgroup of phosphatidylinositol leading to the synthesis of second messengers PtdIns3P, PtdIns(3,4)P2, PtdIns(3,5)P2 and PtdIns(3,4,5)P3. The p110α and p110β are the main forms in insulin target tissues. Especially, p110α isoform (PIK3CA) plays the predominant role in insulin signaling. In particular, PIK-75 is a p110α selective inhibitor.
PIK-75 potently blocks the production of PI(3,4)P2and PIP3 in adipocytes and PIP3 in myotubes. Treatment with the p110α inhibitor PIK-75 dramatically reduces the overall level of PI3K activity in immunoprecipitates from both cells. PIK-75 blocks the phosphorylation of PKB induced by insulin on both Ser473 and Thr308 in CHO-IR cells in a dose-dependent manner, with an IC50 of 78 nM. In 3T3-L1 pre-adipocytes, inhibition of p110α by PIK-75 decreases the insulin-induced phosphorylation of PKB on both sites (Ser473 and Thr308). In J774.2 macrophage cells, insulin strongly increases the phosphorylation of PKB. PIK-75 partially attenuates insulin-induced phosphorylation of Ser473 of PKB. PIK-75 blocks phosphorylation of Akt and arrests cells and induces apoptosis.
In addition, PIK-75 is a potent inhibitor of NRF2 by inducing its proteasomal degradation in human pancreatic cancer cells. PIK-75 also acts as an agent to down-regulate the NRF2 protein level. Moreover, PIK-75 potentiates Gemcitabine-induced antitumor effect through the downregulation of MRP5 in vitro. The combination of PIK-75 with Gemcitabine reduces in vivo tumor growth of human pancreatic cancer. Furthermore, PIK-75 (2 mg/kg) potentiates the anticancer activity of Gemcitabine in vivo.
In summary, PIK-75, targeting the PI3K p110α isoform, impairs cell proliferation, survival, and tumor growth.