The ubiquitin-proteasome-system (UPS) is responsible for most of the intracellular protein degradation in eukaryotes. UPS consists of three components: the proteasomes, the ubiquitin conjugating system, and the deubiquitinating enzymes (DUBs). DUBs deconjugate ubiquitin from targeted proteins and this step is essential for protein degradation. The de-ubiquitination is required for regulating proteasome-mediated protein degradation. The human genome encodes for ~100 DUBs. DUBs have two families; cysteine proteases and metalloproteases. Within the cysteine-based DUB family, Ubiquitin-Specific Proteases (USPs) and Ubiquitin C-terminal Hydrolases constitute >90% of the mammalian cell DUBs pool. Importantly, a number of cancer types differentially express and activate the members of both USP and UCH families. In addition, their aberrant activity links to cancer progression and the onset of chemoresistance. Thus, DUBs is a potential therapeutic target for cancer treatment. In this study, RA-9 is a cell-permeable, potent and specific inhibitor of the subset of deubiquitinating enzymes.
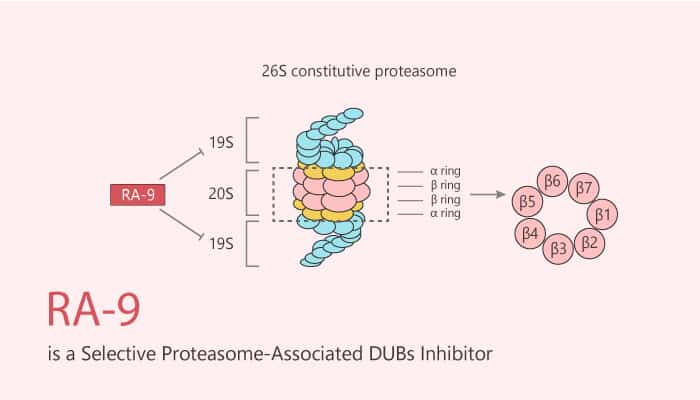
RA-9 is a potent and selective proteasome-associated DUBs inhibitor with favorable toxicity profile and anticancer activity.
It blocks ubiquitin-dependent protein degradation without impacting 20S proteasome proteolytic activity. It also selectively induces the onset of apoptosis in ovarian cancer cell lines and primary cultures derived from donors. In addition, it induces endoplasmic reticulum (ER)-stress responses in ovarian cancer cells. Moreover, RA-9 inhibits the growth of ovarian cancer cell lines and primary cultures. It also causes cell cycle arrest and caspase-mediated apoptosis in ovarian cancer cells. RA-9 also induces ER-stress responses in ovarian cancer cells. Moreover, it results in time-dependent accumulation of the cleaved formed of PARP noticeable as early as 8 hours. Furthermore, RA-9 inhibits human ovarian cancer cell growth in vivo and prolongs survival in a mouse model for ovarian cancer.
In summary, RA-9 is an inhibitor of proteasome-associated DUBs. It inhibits proteasome-associated DUBs and ovarian cancer in vitro and in vivo via exacerbating unfolded protein responses.
Reference:
Coughlin K, et al. Clin Cancer Res. 2014;20(12):3174-3186.