Bcl-2 and Bcl-xL are critical regulators of apoptosis. The pharmacological inhibition of Bcl-2 and Bcl-xL represents a promising strategy for cancer treatment. In addition, BM-1197 is a potent and efficacious dual inhibitor of Bcl-2 and Bcl-xL. BM-1197 binds to Bcl-2 and Bcl-xL proteins with Ki values less than 1 nM and shows >1,000-fold selectivity over Mcl-1.
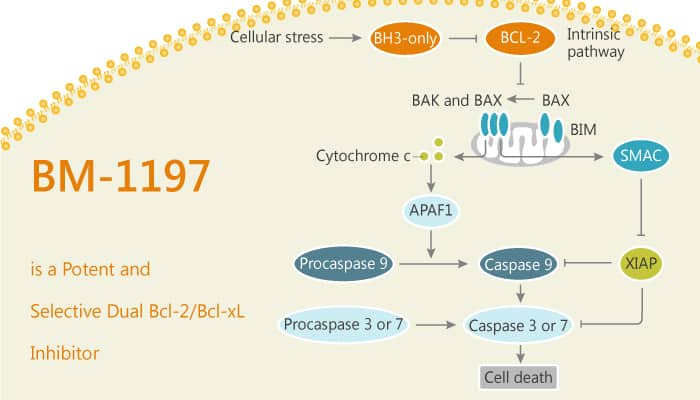
BM-1197 potently disassociates the heterodimeric interactions between anti-apoptotic and pro-apoptotic Bcl-2 family proteins. Moreover, BM-1197 exerts potent growth-inhibitory activity in 7 of 12 small cell lung cancer cell lines tested and induces mechanism-based apoptotic cell death. When intravenously administered at daily or weekly in H146 and H1963 small-cell lung cancer xenograft models, BM-1197 achieves complete and long-term tumor regression. Consistent with its targeting of Bcl-xL, BM-1197 causes transit platelet reduction in mice. Collectively, BM-1197 is a promising dual Bcl-2/Bcl-xL inhibitor which warrants further investigation as a new anticancer drug.
BM-1197 induces mechanism-based apoptosis in Mcl1-deficient mouse embryonic fibroblast (MEF) cells and induces apoptosis in MEF/MCL1−/− cells. In addition, BM-1197 efficiently stabilized BCL-xL protein at concentrations as low as 16 nM, but higher concentrations of ABT-263 were required to achieve a similar effect to that of BM-1197. BM-1197 disrupts the association between BCL-XL and PUMA in MEF/MCL1−/− cells. Furthermore, BM-1197 induces apoptosis in a strictly Bax/Bak-dependent manner. BM-1197 exerts potent growth-inhibitory activity against small cell lung cancer cells in vitro. BM-1197 exerts potent cell growth inhibitory activity against small cell lung cancer cell lines with IC50 values 100 nM (3–82 nM).
In summary, BM-1197 is a highly potent, efficacious and bona fide Bcl-2/Bcl-xL inhibitor warranting further investigation as an anticancer agent.