Epidermal growth factor receptor (EGFR) is a growth-factor-receptor tyrosine kinase. Elevated levels of the EGFR, and/or its cognate ligands act as a common component of multiple cancer types and appear to promote solid tumor growth. BDTX-189 is a potent and selective inhibitor of allosteric EGFR and HER2 oncogenic mutations.
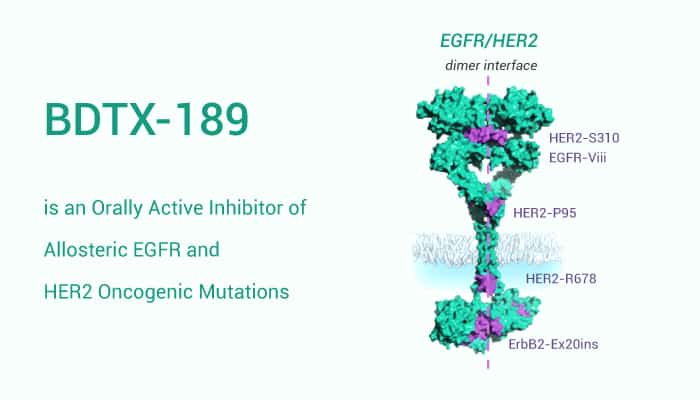
BDTX-189 potently inhibits the family of allosteric EGFR oncogenes, including both extracellular domain mutants and Exon 20 insertion mutants, with greater than 10-fold selectivity versus WT-EGFR. Moreover, BDTX-189 inhibits the activity for a broad range of allosteric EGFR mutants in vivo, including patient-derived xenografts, producing growth regression of allosteric EGFR mutant tumors.
EGFR is one of the most potent oncogenes that are commonly altered in cancers. BDTX-189 potently inhibits allosteric ErbB mutants in vivo and promotes the regression of tumors expressing allosteric ErbB mutations. In addition, BDTX-189 is an ATP-competitive inhibitor of a family of Allo-ErbB oncogenes, including the Allo-ErbB exon 20 insertion family. Besides, BDTX-189 inhibits the growth of and promotes the regression of, Allo-HER2 mutant tumors. BDTX-189 treatment results in rapid regression of EGFR-exon 20 insertion patient-derived xenograft (PDX) tumors. BDTX-189 is designed with rapid clearance to achieve a long biological pharmacodynamic effect while minimizing potential toxicities. Furthermore, BDTX-189 achieves dose-dependent regression of allosteric HER2 and EGFR tumors at well-tolerated doses.
All in all, BDTX-189 is a small molecule ATP-competitive inhibitor against a family of allosteric EGFR and HER2 mutations. BDTX-189 potently inhibits the proliferation of cells driven by allosteric ErbB oncogenes, with selectivity versus cells expressing WT-EGFR.