The cell cycle is a highly conserved process. Additionally, this process must maintain genomic integrity and replicative capacity for proper cell maintenance and proliferation.
The cell cycle consists of four distinct phases: G1 or Gap1 phase, G2 or Gap2 phase, and M phase. As we all know, the G1 phase is responsible for cell growth and synthesizes proteins in preparation for DNA synthesis. And in the S phase, where DNA synthesis occurs. In the G2 phase post-synthesis, cells continue to synthesize proteins in order to increase mass in preparation for mitosis. Lastly, the M phase in which the DNA divides and the parent cell undergoes cytokinesis to produce two daughter cells.
In this article, we will introduce a potent CDK4 and CDK6 inhibitor, Trilaciclib.
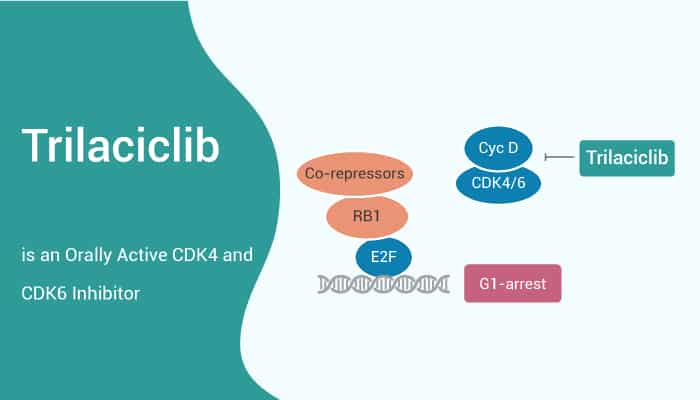
The IC50 values of Trilaciclib are 1 nM and 4 nM for CDK4 and CDK6, respectively. Trilaciclib can induce a robust G1 cell-cycle arrest after 24 hours. Additionally, after 16 hours of Trilaciclib washout, cells reenter the cell cycle and demonstrate cell-cycle kinetics similar to untreated control cells. These results demonstrate that Trilaciclib causes a transient, and reversible G1 arrest.
A transient Trilaciclib-mediated G1 cell-cycle arrest in CDK4/6-sensitive cells decreases the in vitro toxicity of a variety of commonly used cytotoxic chemotherapy agents associated with myelosuppression. Additionally, Trilaciclib results in a robust and dose-dependent suppression of proliferation in HSPCs at 12 hours.
In vivo, 100 mg/kg Trilaciclib 30 minutes prior to etoposide treatment, exhibits only background levels of caspase-3/7 activity. These data demonstrate that Trilaciclib can protect the bone marrow from chemotherapy-induced apoptosis in vivo.
What’s more, Trilaciclib prior to 5-FU likely decreases 5-FU-induced damaged by chemotherapy in HSPCs, thus accelerating blood count recovery after chemotherapy.
In conclusion, Trilaciclib is a potent CDK4/6 inhibitor. It can be used for the reduction of chemotherapy-induced myelosuppression in vivo.
Reference:
[1]. Bisi JE, et al. Mol Cancer Ther. 2016 May;15(5):783-93.