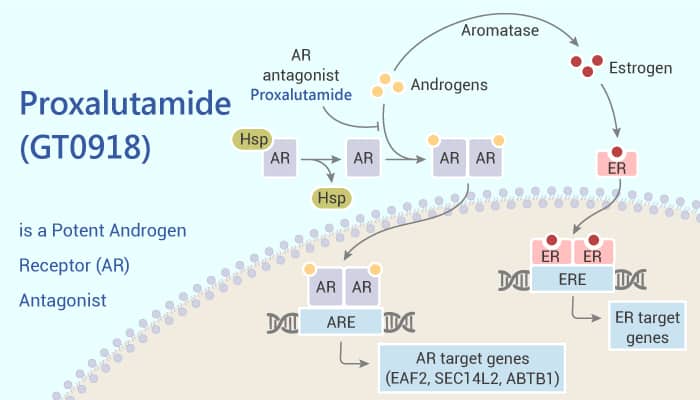
Androgen receptors (ARs) mediate cell differentiation and development of prostate cancer. The target is a common therapeutic target in prostate cancer. Proxalutamide (GT0918) design is based on the core structure of MDV3100 but is optimized by computer-aided design. It is an innovative compound for prostate cancer and advanced breast cancer.
Proxalutamide (GT0918) down-regulates AR protein levels in prostate cancer cells. The inhibitor can overcome the resistance of prostatic cancer cells by downregulating the expression of AR genes. Moreover, Proxalutamide (GT0918, 0-200 μM) dose-dependently inhibits cell viability in LNCaP and 22RV1. Proxalutamide preferentially affected AR-positive PCa cells (IC<sub>50</sub> values from 6.90 to 32.07 μmol/L) over AR-negative cells (IC<sub>50</sub> > 200 μmol/L).
The elimination half-life (t<sub>1/2</sub>) of proxalutamide in rats is approximately 2 h regardless of whether it is administered by the intragastric or the intravenous route. The maximum plasma concentration of proxalutamide (Cmax) could reach 2 μg/mL or higher. Also the oral absolute bioavailability (F) was approximately 80%.
Proxalutamide undergoes in a phase III study in metastatic CRPC in China and in a phase II study in the United States. Moreover, the novel ability of proxalutamide to downregulate AR protein levels may enable efficacy inCRPC. CRPC is resistant to traditional AR inhibitor therapy, as AR upregulation remains the main driver of resistance in CRPC. Proxalutamide has now entered phase III clinical trials.
By the way, Proxalutamide Significantly Accelerates Viral Clearance and Reduces Time to Clinical Remission in Patients with Mild to Moderate COVID-19.
To sum up, Proxalutamide (GT0918) is an orally active potent androgen receptor (AR) antagonist. And Proxalutamide (GT0918) plays important roles in the study of prostate cancer and COVID-19.