Histone acetyltransferases (HATs) are epigenetic enzymes that relieve transcriptional repression by preferentially acetylation of ε-amino group of lysine residues on histones. In general, HATs are crucial for chromatin restructuring and transcriptional regulation in eukaryotic cells. HATs can be grouped into at least five different subfamilies (HAT1, Gcn5/PCAF, MYST, p300/CBP, and Rtt109). HATs mediate many different biological processes including cell-cycle progression, dosage compensation, repair of DNA damage, and hormone signaling. Dysregulation of HATs is strongly correlated with etiology of several diseases especially cancer. Thus there is urgent demand to develop potent small molecule inhibitors against this potential therapeutic target.
Recently, Cheng Luo et al. identified DCH36_06 as a potent and selective p300/CBP inhibitor through virtual screening and iterative optimization. And the suppression of p300/CBP activity retarded cell proliferation in several leukemic cell lines. In addition, DCH36_06 significantly decreases global H3K18ac levels in a dose-dependent manner in leukemic cells. Meanwhile, DCH36_06 arrests cell cycle and induces apoptosis in HAT-abundant leukemic cells. Specifically, DCH36_06 dose-dependently arrests cell cycle at G1 phase phenocopying. Furthermore this compound significantly activates the cleavage of pro-caspase 3, pro-caspase 9 and PARP1 at dose-dependent manner. Notably, DCH36_06 altered downstream gene expression and apoptotic pathways-related genes, such as MYC, HIF1A, UHRF1, RRM2B, SESN2, CCNA2, CCNB1, GADD45B, DEPDC1 and BAX. Most importantly, DCH36_06 also has strong antitumor efficacy in vivo, because DCH36_06 inhibits the leukemic xenograft growth in mice.
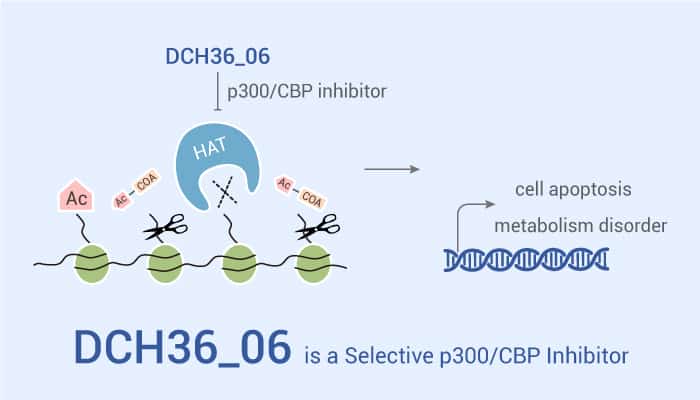
In conclusion, DCH36_06 is a potent and selective p300/CBP inhibitor with strong anti-tumor activities both in vivo and in vitro.
References:
[1] Wenchao Lu, et al. Bioorg Med Chem. 2018 Nov 1;26(20):5397-5407.