The myeloproliferative neoplasms (MPNs) are a group of related clonal diseases probably arising from hematopoietic progenitor or stem cells. Patients with MPNs have an increased risk of thrombotic and bleeding complications and disease progression to acute myeloid leukemia. JAK2 is a member of the JAK family of cytoplasmic tyrosine kinases. They also include JAK1, JAK3, and TYK2. The JAK enzymes are required for signaling by cytokine and growth factor receptors that lack intrinsic kinase activity. JAK1 plays a major role in the signaling of a number of proinflammatory cytokines. It is also often in association with other JAK family members. JAK2 is used primarily by receptors for hematopoietic growth factors. It includes erythropoietin and thrombopoietin (TPO). Constitutive JAK2 activation in hematopoietic cells by the JAK2V617F mutation recapitulates myeloproliferative neoplasm (MPN) phenotypes in mice, establishing JAK2 inhibition as a potential therapeutic strategy. Ruxolitinib is a potent and selective JAK1/2 inhibitor.
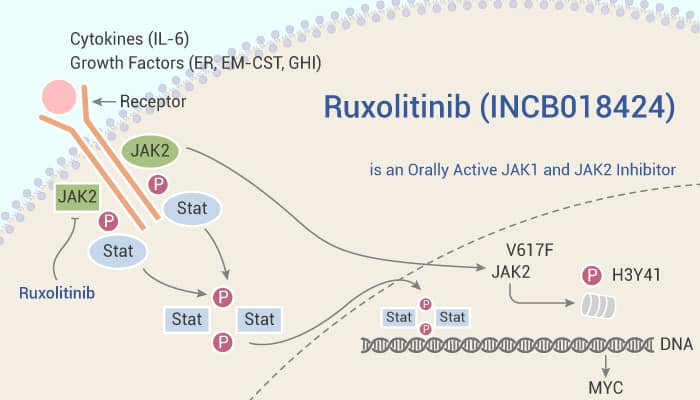
Ruxolitinib potently and selectively inhibits JAK2V617F-mediated signaling and proliferation. It also markedly increases apoptosis in a dose-dependent manner, and at 64 nM results in a doubling of cells with depolarized mitochondria in Ba/F3 cells. In addition, it demonstrates remarkable potency against erythroid colony formation with IC50 of 67 nM and inhibits proliferating of erythroid progenitors from normal donors and polycythemia vera patients with IC50 values of 407 nM and 223 nM, respectively. Moreover, Ruxolitinib results in a survival rate of greater than 90% by day 22 and markedly reduces splenomegaly and circulating levels of inflammatory cytokines, and preferentially eliminated neoplastic cells. It results in significantly prolonged survival without myelosuppressive or immunosuppressive effects in a JAK2V617F-driven mouse model.
In summary, Ruxolitinib is a potent and selective JAK1/2 inhibitor. It is a promising oral agent for the treatment of MPNs.
Reference:
Quintas-Cardama A, et al. Blood, 2010, 115(15), 3109-3117.