mTOR is a member of the phosphatidylinositol 3-kinase related kinase family of protein kinases. Meanwhile, mTOR is linked to other proteins and serves as the core component of two different protein complexes, mTOR complex 1 and mTOR complex 2. They regulate different cellular processes. Nonetheless, mTOR C1 phosphorylates p70 ribosomal protein S6 kinase (P70S6K) and translation initiation regulator 4E binding protein 1 (4E-BP1). It also regulates protein translation and cell proliferation. mTOR C2 phosphorylates Akt and SGK1 and regulates cell survival, apoptosis, and cytoskeletal tissue. Particularly, mTOR is a multifunctional signal transduction protein. It can obtain signals from many upstream pathways, including nutrition supply, and spread information by regulating multiple downstream pathways.
Obviously, mTOR is a part of the phosphatidylinositol 3-kinase/Akt/mTOR signaling pathway and is also related to tumor angiogenesis. This pathway is also becoming a key regulator of hypoxia and growth factor-mediated tumor hypoxia-induced expression. In addition, mTOR is very conserved and widely expressed, including in endothelial cells. In addition to playing a key role in normal cell physiology, mTOR is also associated with cancer. Interestingly, mTOR is a serine/threonine kinase, which plays a key role in cancer cell proliferation and is a promising target for cancer therapy. Here, we will introduce a selective and orally active mTOR1 inhibitor, Everolimus.
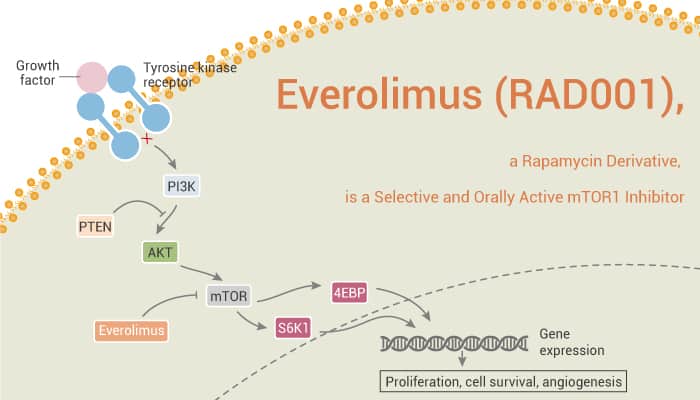
Everolimus (RAD001), a Rapamycin Derivative, is a Selective and Orally Active mTOR1 Inhibitor.
First of all, Everolimus binds to FKBP-12 to generate an immunosuppressive complex. Everolimus inhibits tumor cells proliferation and induces cell apoptosis and autophagy. Specifically, Everolimus has potent immunosuppressive and anticancer activities.
Secondly, Everolimus inhibits the sensitive murine B16/BL6 melanoma (IC50, 0.7 nM). And it inhibits insensitive human cervical KB-31 (IC50, 1,778 nM). Besides, Everolimus results in total dephosphorylation of S6K1 and the substrate S6 and a shift in the mobility of 4E-BP1. This is indicative of reduced phosphorylation status. Moreover, Everolimus exhibits a dose-dependent inhibition in the stem cells from the BT474 cell line and the primary breast cancer cells. The IC50 values of Everolimus for BT474 and the primary CSCs are 2,054 and 3,227 nM, respectively.
Thirdly, daily treatment with Everolimus (0.5 or 2.5 mg/kg) dose-dependently inhibits growth in the rat CA20498 model. Furthermore, a higher dose of 5 mg/kg (once or twice per week) also shows similar antitumor efficacy. With either growth factor, Everolimus dose-dependently increases the hemoglobin content but reduces the Tie-2 content.
Finally, Everolimus (RAD001), a Rapamycin derivative, is a potent, selective, and orally active mTOR1 inhibitor.
References:
Lane HA, et al. Clin Cancer Res, 2009, 15(5), 1612-1622.