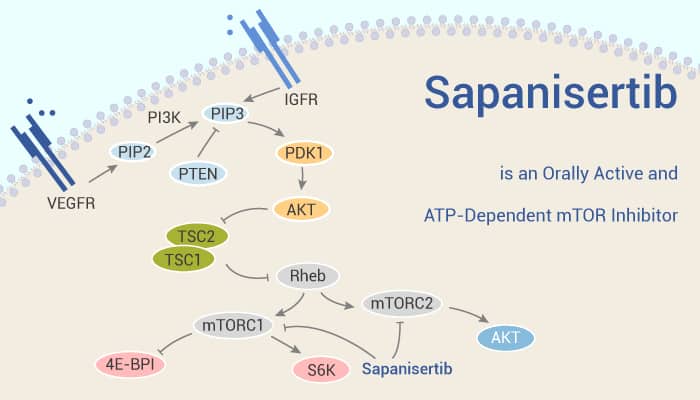
Acute myelogenous leukemia (AML) is a heterogeneous disease. It often relapses after standard chemotherapy or proves refractory to available treatments. Therefore, It needs novel therapies for AML. In AML, many signaling pathways are abnormally activated and lead to uncontrolled proliferation/survival of immature myeloid progenitors. The 50% to 80% of AML cases activate mTOR signaling pathway. mTOR is a serine/threonine protein kinase. It senses signals of growth factors, nutrients, energy status, and metabolic stresses. The mTOR exists in two distinct multi-factor complexes: mTOR complex 1 (mTORC1) and 2 (mTORC2). In this study, Sapanisertib (TAK-228, MLN0128) is a selective, highly potent, and orally bioavailable ATP competitor of both mTORC1 and mTORC2. It is currently in phase I and II clinical trials as a single agent and in combination with other therapeutic agents in patients with advanced malignancies.
Sapanisertib not only decreases pevonedistat induced apoptosis and reduces apoptosis markers. It includes cleaved caspase3/8 and cleaved PARP levels, it also restored anti-apoptosis protein XIAP levels. Sapanisertib alone induces limited cell death in most AML cell lines and primary AML blasts, but it was able to inhibit cell cycle progression, reduce cell size, and protect from apoptosis. Pevonedistat and Sapanisertib, therefore, exhibit distinct anti-tumor effects on AML cells. They are cytotoxic and cytostatic effects, respectively.
In summary, Sapanisertib is an investigational, orally available, potent, and highly selective mTORC1/2 inhibitor demonstrating promise in numerous malignancies. In addition, Pevonedistat, an inhibitor of the NEDD8 activating enzyme (NAE), and Sapanisertib, an inhibitor of mTORC1 and mTORC2 as single agents or in combination on AML cell lines.
Reference:
Guo N, et al. Transl Oncol. 2019;12(4):602-613.