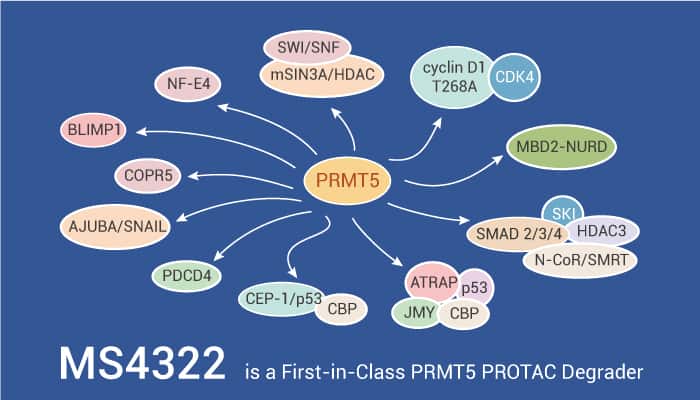
PRMT5 is a member of type II protein arginine methyltransferases (PRMTs). When interacting with its binding partner MEP50, PRMT5 catalyzes monomethylation and symmetric dimethylation of arginine residues of its histone substrates. These substrates include H3R2, H3R8, and H4R3, and its nonhistone substrates, including p53, EGFR, N-MYC, SmD3, and RNA polymerase II.
In this article, we will introduce a first-in-class PRMT5 degrader, MS4322.
In Biochemical assays, The PRMT5 inhibitor inhibits PRMT5 Methyltransferase activity with an IC50 of 18 nM.
Additionally, in MCF-7 cells, MS4322 effectively reduces the PRMT5 protein level in a concentration-dependent manner. The DC50 and Dmax of MS4322 are 1.1±0.6 μM and 74±10%, respectively. compound 15-induced PRMT5 degradation is dependent on the ubiquitin-proteasome system.
MS4322 exhibits an anti-proliferative effect due to its PRMT5 degradation activity, but not its PRMT5 inhibitory activity. And it potently inhibits MCF-7 cell proliferation in a concentration-dependent manner. It is at least as effective as the positive control EPZ015666.
In Vivo Mouse PK Study. A single IP injection results in good exposure levels in plasma. In addition, a very high plasma concentration (14 ± 2 μM) achieves at 2 h post dosing. What’s more, Even after 12 h, the concentration of MS4322 is still above 100 nM.
In addition, MS4322 is well tolerated by the treated mice. Taken together, these results suggest that MS4322 is a valuable tool for investigating the effects of PRMT5 degradation in vivo.
MS4322 is a highly selective PRMT5 degrader and is also the first degrader of any PRMTs, an important subclass of HMTs.
MS4322 effectively reduces the PRMT5 protein level in MCF-7 cells in a concentration-, time-, PRMT5-, VHL-, and proteasome-dependent manner. EPZ015666 at inhibiting global SDMA and proliferation in MCF-7 cells.
In addition, it is able to significantly reduce PRMT5 protein levels in other cancer lines. Collectively, MS4322 is a promising compound for further exploring PRMT5 functions in physiology and pathophysiology.
[1]. Shen Y, et al. J Med Chem. 2020 Sep 10;63(17):9977-9989.