Lung cancer is one of the most common malignant tumors in the world. Its lethality ranks the first among many malignant tumors. In addition, the overexpression of the human ATP-binding cassette (ABC) drug transporter ABCB1 (P-gp) or ABCG2 (BCRP) in cancer cells often contributes significantly to the development of multidrug resistance (MDR) in cancer patients. Some EGFR tyrosine kinase inhibitors (TKIs) could modulate the activity of ABCB1 and/or ABCG2 in human cancer cells, whereas some EGFR TKIs are transport substrates of these transporters. In this study, Almonertinib (HS-10296) is a novel, third-generation EGFR tyrosine kinase inhibitor. It targets both EGFR-sensitizing and T790M resistance mutations. More importantly, Almonertinib is able to reverse MDR mediated by ABCB1 in multidrug-resistant cancer cells at submicromolar concentrations. It inhibits the drug transport activity of ABCB1 without affecting its expression level.
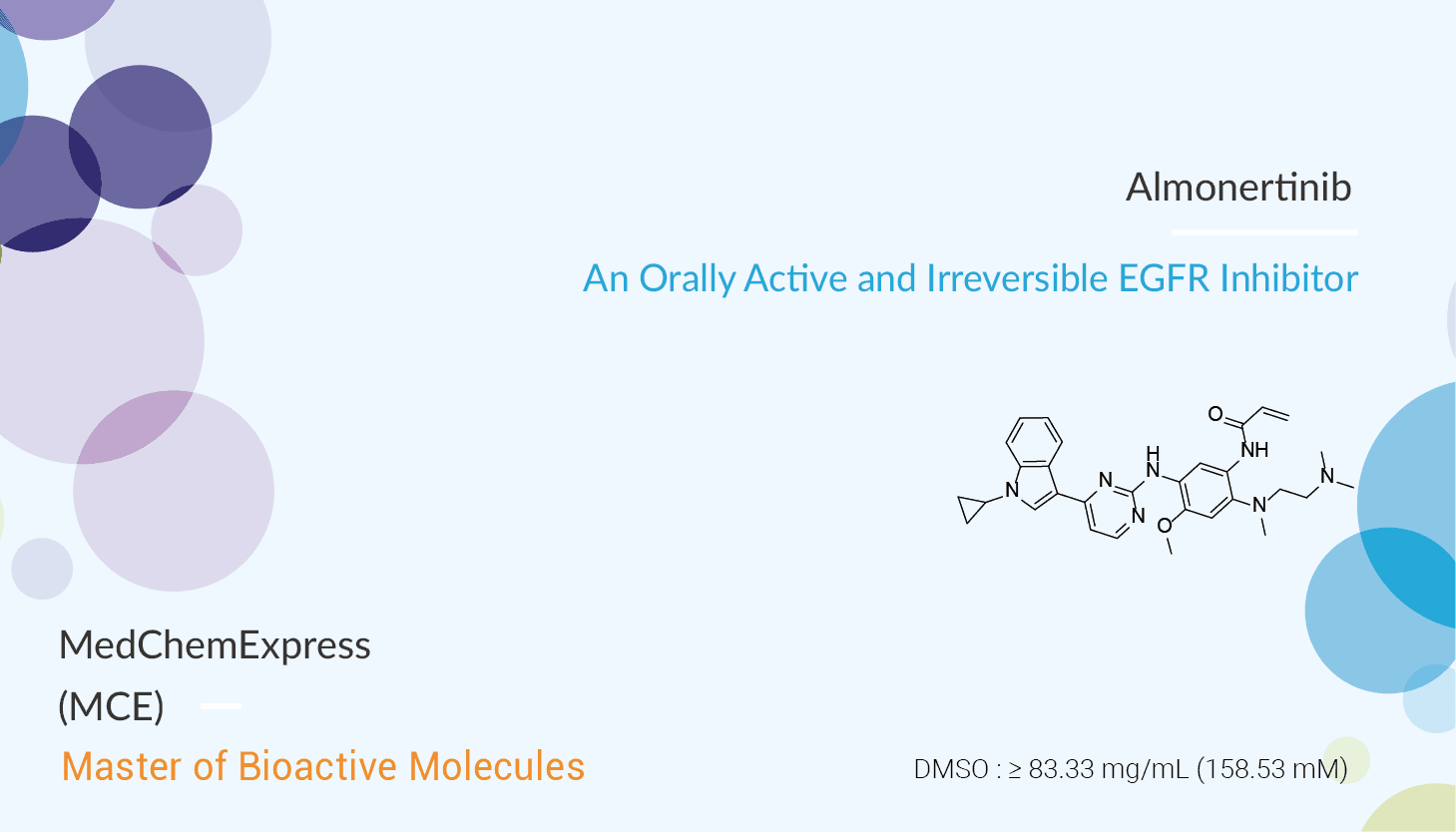
Almonertinib is an orally available, third-generation EGFR TKI. Moreover, it reveals great inhibitory activity against T790M, T790M, and L858R, and T790M and Del19. The IC50s are 0.37, 0.29, and 0.21 nM, respectively. It is less effective against WT. In addition, Almonertinib (20 mg/kg; orally once daily) for 14 days inhibits the growth of transplanted National Cancer Institute-H1975 tumor cells (L858R and T790M mutation-positive) in eight of eight mice, and the tumor growth inhibition rate is comparable with that of Osimertinib. In addition, Almonertinib resensitizes ABCB1-overexpressing multidrug-resistant cancer cells to chemotherapeutic drugs. Almonertinib significantly reverses ABCB1-mediated resistance to its substrates paclitaxel , vincristine, and colchicine in ABCB1-overexpressing NCI-ADR-RES, KB-V-1, and MDR19 cell lines at submicromolar concentrations.
In summary, Almonertinib is an orally available, irreversible, third-generation EGFR tyrosine kinase inhibitor. Almonertinib resensitizes ABCB1-overexpressing multidrug-resistant cancer cells to chemotherapeutic drugs.
Reference:
Yang JC, et al. Safety, J Thorac Oncol. 2020;15(12):1907-1918.; Wu CP, et al. Biochem Pharmacol. 2021;188:114516.