Phospholipase is a member of a very complex group of enzymes that break down phospholipids into fatty acids and other compounds. Phospholipase D (PLD) is an enzyme of the phospholipase superfamily. There are two mammalian isoforms of PLD, coined PLD1 and PLD2, and despite conserved regulatory and catalytic domains. Moreover, plants contain numerous genes that encode various PLD isoenzymes, with molecular weights ranging from 90 to 125 kDa. Importantly, PLD is an important player in many physiological processes, including membrane trafficking, cytoskeletal reorganization, receptor-mediated endocytosis, exocytosis, and cell migration. Besides, PLD has been implicated in a human cancer cell progression (breast, renal, gastric and colorectal) as well as actin cytoskeleton reorganization and cell motility.
VU0359595 (also known as CID-53361951 or ML-270) is a potent and selective phospholipase D1 (PLD1) inhibitor. VU0359595 is >1700-fold selective for PLD1 over PLD2. This compound has various biological activities. For example, VU0359595 inhibits basal and FCS/IGF-1 stimulated proliferation of astroglial cells. Meanwhile, it does not affect basal PLD activity in astrocytes but reduces mitogen-stimulated PLD activity in a concentration-dependent manner. In addition, VU0359595 partially reduces the increase [3H]-phosphatidylethanol generation induced by high glucose in retinal pigment epithelium cells. VU0359595 prevents the loss in cell viability induced by LPS (10 μg/mL) in D407 cells. Furthermore, VU0359595 also increases autophagosome-like structures in cells exposed to LPS.
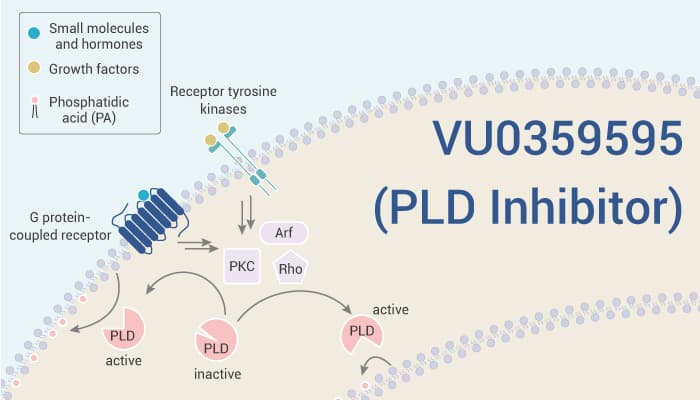
To sum up, VU0359595 is a potent and selective PLD1 inhibitor that shows >1700-fold selective for PLD1 over PLD2.
References:
[1] Jana A Lewis, et al. Bioorg Med Chem Lett. 2009 Apr 1;19(7):1916-20.
[2] Vicente Bermúdez, et al. Front Cell Neurosci. 2019 Apr 24;13:154.