Pirarubicin has widely application in intravesical chemotherapy for bladder cancer, but drug resistance limits its efficacy; the mechanism still needs further exploration.
Bladder cancer is the most common malignancy of the urinary tract in China. 75–85% of bladder cancers are grouped as non-muscle invasive bladder tumors. Intravesical chemotherapy is the standard adjuvant treatment of non-muscle invasive bladder cancer. Pirarubicin (also known as THP) has been widely used in intravesical chemotherapy and decreased the tumor recurrence rate significantly, but 10–30% of patients still relapse and metastasize within 5 years. Previous studies have demonstrated that pirarubicin can interfere with transcription and prevent DNA synthesis.
Pirarubicin can induce autophagy in bladder cancer cells, a cytoprotective response against pirarubicin-induced cytotoxic effects. Furthermore, mTOR/p70S6K/4E-BP1 signaling pathway plays a major role in the regulation of autophagy in pirarubicin-treated bladder cancer cells.
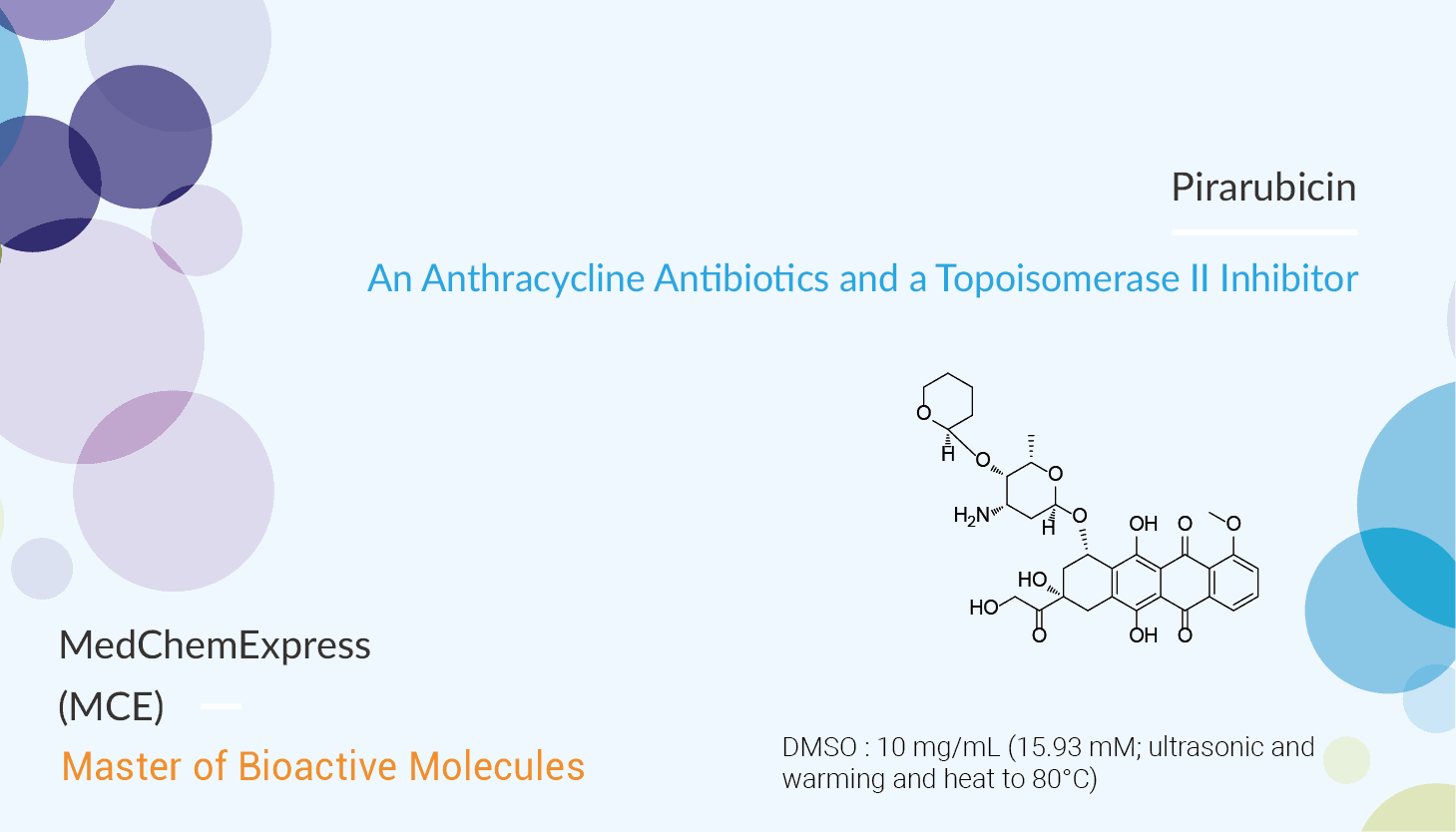
In this article, we will introduce an anti-cancer agent Pirarubicin.
Pirarubicin is an anthracycline antibiotic and also a potent inhibitor of Topoisomerase II.
Pirarubicin is a topoisomerase II inhibitor. This compound shows inhibitory activities against M5076 and Ehrlich cells, with IC50s of 0.366 and 0.078 μM, respectively. The cytotoxicity of Pirarubicin toward M5076 cells is lower than toward Ehrlich cells, and this is due to the much lower expression of topoisomerase II in M5076 cells than in Ehrlich cells. Pirarubicin (2.5, 5, 10 μg/mL) significantly induces autophagy in a dose dependent manner in bladder cancer (T24, EJ, 5637, J82 and UM-UC-3) cells. Furthermore, Pirarubicin (5 μg/mL) induces apoptosis through inhibition of mTOR/p70S6K/4E-BP1 in bladder cancer cells, and this effect is stronger by inhibition of autophagy.
Pirarubicin (18 mg/kg, i.v.) significantly elevates serum level of BNP, CK-MB, CTnT, LDH, and MDA compared with those in the control group in acute cardiac toxicity rats. Pirarubicin also lowers heart rate, and depresses R-wave voltage, and prolongation of QT intervals in the acute cardiac toxicity model.
In conclusion, pirarubicin has the potential for the research of cancer disease including bladder cancer. These findings enhance the understanding of drug resistance mechanisms during treatment of bladder cancer with pirarubicin.
Reference:
Wang YD, et al. Cardioprotective effects of rutin in rats exposed to pirarubicin toxicity.